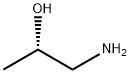
(S)-(+)-1-Amino-2-propanol synthesis
- Product Name:(S)-(+)-1-Amino-2-propanol
- CAS Number:2799-17-9
- Molecular formula:C3H9NO
- Molecular Weight:75.11

2043-43-8
73 suppliers
$15.00/5mg

2799-17-9
237 suppliers
$8.00/1g
Yield:-
Reaction Conditions:
Stage #1:(S)-(-)-lactamide with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 55;
Stage #2: with water in tetrahydrofuran at 0;
Steps:
8
Test Example 8Production of Optically Active Amino Alcohol Using Optically Active Hydroxy EsterThe optically active hydroxy ester obtained according to the production method of the present invention is useful also in producing an optically active amino alcohol. One example of the method for producing an optically active amino alcohol is shown below. As shown in the above reaction scheme, ammonia gas was bubbled into a solution of benzyl (S)-lactate ester (45.2 mg, 0.251 mmol) in methanol (2.50 mL) at 0° C. for 70 min. After the reaction mixture was stirred at room temperature for 11 hrs, concentration gave a crude product of corresponding amide.Subsequently, to a solution of thus obtained crude amide product in tetrahydrofuran (2.50 mL) was added lithium aluminium hydride (92.6 mg, 2.47 mmol) at 0° C. The reaction mixture was stirred at room temperature for 10 min, and the temperature was elevated to 55° C., followed by stirring the same for 18 hrs. Thereafter, water (200 μL) was added at 0° C. to stop the reaction. The reaction mixture was filtrated through Celite, and subjected to vacuum concentration. Thereafter, fractionation was carried out on silica gel thin layer chromatography (developing solvent: chloroform saturated with ammonia by aqueous ammonia extraction/methanol=50/1) to afford corresponding optically active amino alcohol (14.5 mg, 65%). The physical properties were as in the following.[α]D22=+44.4 (c 1.0, methanol);1H NMR (CDCl3): δ3.65-2.55 (m, 1H), 3.80-2.40 (br m, 3H), 2.65 (dd, J=12.7, 2.7 Hz, 1H), 2.44 (dd, J=12.7, 8.1 Hz, 1H), 1.06 (d, J=6.3 Hz, 3H).
References:
Tokyo University of Science Educational Foundation Administrative Organization US2011/319650, 2011, A1 Location in patent:Page/Page column 18