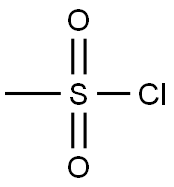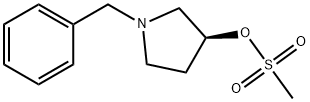
(S)-1-BENZYL-3-MESYLOXY PYRROLIDINE synthesis
- Product Name:(S)-1-BENZYL-3-MESYLOXY PYRROLIDINE
- CAS Number:118354-71-5
- Molecular formula:C12H17NO3S
- Molecular Weight:255.33
Yield:118354-71-5 99%
Reaction Conditions:
with triethylamine in toluene at 0 - 20; for 3.91667 h;
Steps:
5.1
EXAMPLE 5. SYNTHESIS OF I-METHYL-I-PYRROLIDIN-3-YL ETHYLAMINE (5); 1 -Methyl- l-pyrrolidin-3-yl-ethylamine is prepared in accordance with the synthetic scheme below.N O5 PStep 1.; Synthesis of (S)-l-benzylpyrrolidin-3-yl methanesulfonate (N).[0258]; Methanesulfonyl chloride (15 mL, 0.19 mol) is added to a cooled (0 0C) solution of toluene (300 mL) containing (5)-l-benzylpyrrolidin-3-ol (24.5 g, 0.14 mol) and triethylamine (80 mL, 0.57 mol). The resulting mixture is stirred at 0 °C for 15 min, and allowed to warm to room temperature with stirring for 2h. The mixture is quenched with a 5% aqueous solution of sodium bicarbonate (250 mL). The organic layer is washed with a 5% aqueous solution of sodium bicarbonate (2 x 250 mL), washed with water (I x 250 mL), dried over magnesium sulfate, and concentrated under reduced pressure to give N (35.1 g, 99 %) as an orange oil. 1H NMR (300 MHz, CDCl3): £2.07 (m, IH), 2.30 (m, IH), 2.49 (m, IH), 2.75-2.90 (m, 3H), 2.98 (s, 3H), 3.61 (d, J= 13.0 Hz, IH), 3.68 (d, J= 13.0 Hz, IH), 5.18 (m, IH), 7.15-7.30 (m, 5H). LCMS mlz calcd for C12H17NO3S 255 ([M+]); found 256 ([M + H]+, 100%), 160 (40%).
References:
WO2007/14308,2007,A1 Location in patent:Page/Page column 70
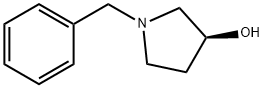
101385-90-4
299 suppliers
$12.00/1g

118354-71-5
20 suppliers
inquiry
![1,4-Butanediol, 2-[bis(phenylmethyl)amino]-, (2R)-](/CAS/20210305/GIF/908598-98-1.gif)
908598-98-1
0 suppliers
inquiry

118354-71-5
20 suppliers
inquiry
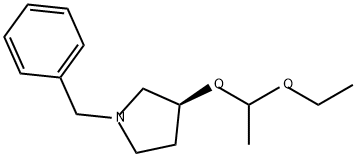
188790-86-5
0 suppliers
inquiry

118354-71-5
20 suppliers
inquiry