
(S)-1-(TETRAHYDRO-2H-PYRAN-4-YL)ETHANAMINE synthesis
- Product Name:(S)-1-(TETRAHYDRO-2H-PYRAN-4-YL)ETHANAMINE
- CAS Number:1269754-98-4
- Molecular formula:C7H15NO
- Molecular Weight:129.2
![2-Propanesulfinamide, 2-methyl-N-[(1S)-1-(tetrahydro-2H-pyran-4-yl)ethyl]-, [S(R)]-](/CAS/20210305/GIF/1269755-00-1.gif)
1269755-00-1
0 suppliers
inquiry

1269754-98-4
26 suppliers
$45.00/2.5mg
Yield:-
Reaction Conditions:
Stage #1: (R)-2-methyl-N-[(1S)-1-(oxan-4-yl)ethyl]propane-2-sulfinamidewith hydrogenchloride in 1,4-dioxane;methanol at 20; for 0.5 h;
Stage #2: with sodium hydrogencarbonate in water;
Steps:
3
To a solution of (R)-2-methyl-N-((S)-l-(tetrahydro-2H-pyran-4- yl)ethyl)propane -2- sulfanamide (400 mg, 1.714 mmol) in MeOH (5 mL) was added 4M hydrochloride in dioxane (5 mL). The reaction mixture was stirred at room temperature for 30 min. The mixture was concentrated under reduced pressure and the residue was diluted with diethylether (10 mL). The precipitate was collected by filtration and washed with diethylether providing crude (S)-l-(tetrahydro-2H-pyran-4-yl)ethanamine hydrochloride salt. The hydrochloride salt was dissolved in water (10 mL) and neutralized with saturated aqueous sodium bicarbonate solution. The mixture was extracted with dichloromethane. The organic layer was dried over sodium sulfate, filtered off and concentrated under reduced pressure providing crude (S)- 1 -(tetrahydro- 2H-pyran-4-yl)ethanamine (212 mg), which was directly used in the next reaction without further purification. LCMS (m/z): 130.1 [M+H]+; Rt = 0.34 min.
References:
WO2011/26917,2011,A1 Location in patent:Page/Page column 56

1269756-02-6
21 suppliers
inquiry

1269754-98-4
26 suppliers
$45.00/2.5mg
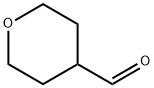
50675-18-8
210 suppliers
$15.00/100mg

1269754-98-4
26 suppliers
$45.00/2.5mg
![2-Propanesulfinamide, 2-methyl-N-[(tetrahydro-2H-pyran-4-yl)methylene]-, [N(E),S(R)]-](/CAS/20240320/GIF/1269754-99-5.gif)
1269754-99-5
1 suppliers
inquiry

1269754-98-4
26 suppliers
$45.00/2.5mg
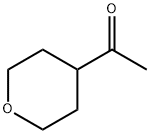
137052-08-5
193 suppliers
$12.00/1g

1269754-98-4
26 suppliers
$45.00/2.5mg