(S)-2,2-DIMETHYL-1,3-DIOXOLANE-4-ACETAMIDE synthesis
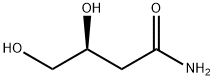
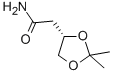
- Product Name:(S)-2,2-DIMETHYL-1,3-DIOXOLANE-4-ACETAMIDE
- CAS Number:185996-33-2
- Molecular formula:C7H13NO3
- Molecular Weight:159.18
Yield:185996-33-2 100%
Reaction Conditions:
Stage #1: 2,2-dimethoxy-propane;(S)-3,4-dihydroxybutyramide;sulfuric acid in acetone at 20 - 60; for 12.5 h;
Stage #2: with silver(l) oxide in acetone; for 1 h;Product distribution / selectivity;
Steps:
1; 2
(S)-3-hydroxy-γ-butyrolactone (1) (51 g, 0.5 mol) was converted to the amide (2) by treatment at room temperature for 14 hours with 110 ml of 30% ammonium hydroxide (0.85 mol). The solution was then concentrated to a syrup at ~50°C under reduced pressure until no more water could be removed. Acetone (500 mL) and 2,2-dimethoxypropane (104 g, 1 mol) was added. Sulfuric acid (2 mL) was then added and the mixture protected from moisture with a calcium chloride drying tube, heated at 60°C for 30 minutes and stirred at room temperature for 12 hours. Silver oxide (20 g) was added and the mixture stirred for 1 hour. Methanol (200 ml) was then added and the mixture filtered and concentrated to dryness. The protected amide (3) was crystallized upon concentrating and was used directly in the next step. Conversion to the protected amide (3) was essentially quantitative. A small amount when recrystallized from acetone gave white crystals mp, 98-100°C. [α]589 = -15.4 (CHCl3, c = 1), 1H-NMR (CDCl3, 300 MHz) δ 6.10 (s, 1H), 5.65 (s, 1H), 4.43 (m, 1H), 4.14 (dd, 1H, J=8.1 and 6.3 Hz) 3.63 (dd, 1H, J=8.1 and 6.8 Hz) 2.55 (dd, 1H, J=15.3 and 7.5 Hz), 2.46 (dd, 1H, J=15.3 and 4.8 Hz), 1.42 (s, 3H), 1.35 (s, 3H) 13C-NMR (CDCl3 75 MHZ) δ 172.86, 109.50, 72.21, 69.05, 40.07, 26.90, 25.50.
References:
EP1086072,2006,B1 Location in patent:Page/Page column 7; 8-9

95422-24-5
28 suppliers
inquiry

185996-33-2
13 suppliers
inquiry