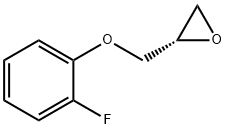
(S)-2-((2-FLUOROPHENOXY)METHYL)OXIRANE synthesis
- Product Name:(S)-2-((2-FLUOROPHENOXY)METHYL)OXIRANE
- CAS Number:184488-19-5
- Molecular formula:C9H9FO2
- Molecular Weight:168.16
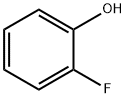
367-12-4
418 suppliers
$5.00/10g

57044-25-4
250 suppliers
$6.00/1g

184488-19-5
12 suppliers
$495.18/5MG
Yield:184488-19-5 65%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 0 - 20; for 16 h;
Steps:
9.A
2-fluorophenol (3.00 g, 26.77 mmol) was added to a 100 mL flame-dried round bottom flask followed by (R)-glycidol (2.00 g, 17.8 mmol) and triphenylphosphine (5.61 g, 21.4 mmol). Tetrahydrofuran (90 mL) was the added to the above mixture and the contents were stirred to dissolve. The flask was then cooled in an ice bath and diisopropylazodicarboxylate (5.4 g, 26.7 mmol) was added drop-wise to the above mixture. The solution was stirred at room temperature under nitrogen for 16 hours after which it was concentrated and the crude oil subjected to silica gel flash chromatography (9: 1 pentane: ether) to obtain the title compound (1.95 g, 65%) as a white solid. 1H NMR (600 MHz, CDCl3): δ 7.09-6.98 (m, 3H), 6.93-6.90 (m, IH), 4.26 (dd, IH, J = 11.4, 3.0 Hz), 4.03 (dd, IH, J = 11.1, 6.0 Hz), 3.37-3.35 (m, IH), 2.89 (dd, IH. J= 3.6, 5.1 Hz), 2.75 (dd, IH, J = 3.6, 5.1 Hz); 13C NMR (150 MHz, CDCl3): δ 154.5, 151.2, 146.6, 124.3, 122.01, 116, 70.46, 50.0, 44.6.
References:
WO2008/83054,2008,A2 Location in patent:Page/Page column 39
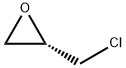
67843-74-7
479 suppliers
$10.00/1g

367-12-4
418 suppliers
$5.00/10g

184488-19-5
12 suppliers
$495.18/5MG