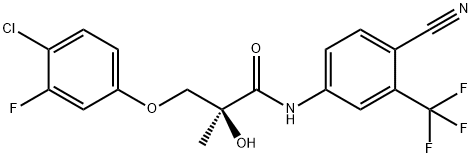
S-23 synthesis
- Product Name:S-23
- CAS Number:1010396-29-8
- Molecular formula:C18H13ClF4N2O3
- Molecular Weight:416.75
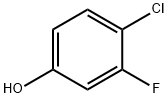
348-60-7
193 suppliers
$8.00/1g
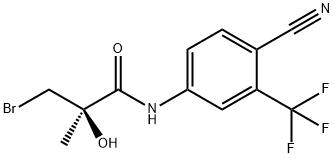
206193-17-1
22 suppliers
inquiry

1010396-29-8
129 suppliers
$49.00/5mg
Yield:1010396-29-8 70.5%
Reaction Conditions:
Stage #1:(2R)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide with potassium carbonate for 2 h;Reflux;
Stage #2:4-chloro-3-fluorophenol with potassium carbonate in isopropyl alcohol for 3 h;Reflux;
Steps:
15 Synthesis of (5)-3-(4-chloro-3-fluorophenoxy)- V-(4-cyano-3- (trifluoromethyl)phenyl)-2-hydroxy-2-methylpropanamide (Formula XIV).
Synthesis of (5)-3-(4-chloro-3-fluorophenoxy)- V-(4-cyano-3- (trifluoromethyl)phenyl)-2-hydroxy-2-methylpropanamide (Formula XIV). A mixture of bromoamide ((2/?)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2- hydroxy-2-methylpropanamide, (R-19) 2.0 g, 5.70 mmol), anhydrous K2CO3 (2.4 g, 17.1 mmol) was heated to reflux for 2 h and then concentrated under reduced pressure to give a solid. The resulting solid was treated with 4-chloro-3-fluorophenol (1.3 g, 8.5 mmol) and anhydrous K2CO3 (1.6 g, 11.4 mmol) in 50 mL of 2-propanol was heated to reflux for 3 h and then concentrated under reduced pressure to give a solid. The residue was treated with 100 mL of H20 and then extracted with EtOAc (2 X 100 mL). The combined EtOAc extracts were washed with 10% NaOH (4 X 100 mL) and brine, successively. The organic layer was dried over MgS04 and then concentrated under reduced pressure to give an oil which was purified by column chromatography using EtOAc/hexane (50:50) to give a solid which was recrystallized from CH2Ci2/hexane to give 1.7 g (70.5%) of (5')-3-(4- chloro-3-fluorophenoxy)-N-(4-cyano-3-(trifluoromethyl)phenyl)-2-hydroxy-2- methylpropanamide as a colorless solid. [000321] FontWeight="Bold" FontSize="10" H NMR (CDCI3/TMS) δ 1.60 (s, 3H, CH3), 3.28 (s, 1H, OH), 3.98 (d, J = 9.05 Hz, 1H, CH), 6.64 - 6.76 (m, 2Η, ArH), 7.30 (d, J = 8.67 Hz, 1H, ArH), 7.81 (d, J = 8.52 Hz, 1H, ArH), 7.96 (q, / = 2.07, 8.52 Hz, 1H, ArH), 8.10 (d, / = 2.07 Hz, 1H, ArH), 9.10 (s, 1Η, NH). Calculated Mass: [Μ-Η]" 414.9. Mp: 132-134 °C.
References:
GTX, INC.;DALTON, James, T.;STEINER, Mitchell, S.;NARAYANAN, Ramesh;AHN, Sunjoo WO2014/11220, 2014, A2 Location in patent:Paragraph 000320-000321
654-70-6
461 suppliers
$5.00/1g

1010396-29-8
129 suppliers
$49.00/5mg