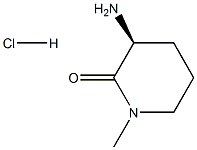
(S)-3-aMino-1-Methylpiperidin-2-one hydrochloride synthesis
- Product Name:(S)-3-aMino-1-Methylpiperidin-2-one hydrochloride
- CAS Number:956109-56-1
- Molecular formula:C6H13ClN2O
- Molecular Weight:164.6332
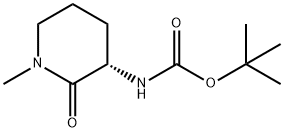
956109-52-7
4 suppliers
inquiry

956109-56-1
44 suppliers
$180.00/50mg
Yield:956109-56-1 100%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 16 h;
Steps:
20.A References:
WO2007/126871,2007,A1 Location in patent:Page/Page column 71
Example 20; Preparation of (SV5-(2,4-difluorophenoxy)-l -isobutyl-iV-d -methyl-2-oxopiperidin-3-ylV lH-indazole-6-carboxamide (Ie)[00266] Step A: Synthesis of fιSV3-ammo-l-methylpiperidin-2-one hydrochloride:(S)-tert-Butyl l-methyl-2-oxopiperidin-3-ylcarbamate (prepared according to Example 12; 78 mg, 0.34 mmol) was dissolved in 4M hydrogen chloride in dioxane (1.7 mL, 6.8 mmol), and the reaction was allowed to stir at ambient temperature for 16 hours. The reaction mixture was concentrated . to yield (