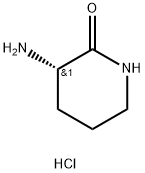
(S)-3-aminopiperidin-2-one Hydrochloride synthesis
- Product Name:(S)-3-aminopiperidin-2-one Hydrochloride
- CAS Number:42538-31-8
- Molecular formula:C5H11ClN2O
- Molecular Weight:150.61
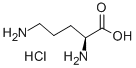
3184-13-2
606 suppliers
$5.00/250mg

42538-31-8
115 suppliers
$47.00/250mg
Yield: 97%
Reaction Conditions:
Stage #1:L-ornithine hydrochloride with chloro-trimethyl-silane in methanol at 20; under 760.051 Torr; for 12 h;
Stage #2: with sodium ethanolate in methanol;ethanol at 0 - 20; under 760.051 Torr; for 0.583333 h;enantioselective reaction;
Steps:
(S)-3-Aminopiperidin-2-one hydrochloride (4)
Trimethylchlorosilane (2.8 mL, 23 mmol, 4 equiv) was added to L-ornithine·HCl (1.0 g,6.0 mmol, 1 equiv) followed by the addition of anhydrous methanol (20 mL). The mixturestirred at rt for 12 h. The solution was then cooled to 0 °C and a 21% (w/w) solution ofsodium ethoxide in ethanol (42 mmol, 17 mL) was added; after 5 min the solution wasallowed to warm to rt and stirred for another 30 min. The solution was neutralized to pH7 with 6 N aq HCl. The resulting solution was filtered and conc. in vacuo. Salts wereremoved by dissolution in isopropanol, filtered, and conc. in vacuo. The crude residuewas purified by flash column chromatography on silica gel (30% methanol indichloromethane) to afford lactam hydrochloride 4 as a hygroscopic, pale yellow solid.
References:
Barnett, Robert;Raszkowski, Daniel;Winckler, Thomas;Stallforth, Pierre [Beilstein Journal of Organic Chemistry,2017,vol. 13,p. 247 - 250] Location in patent:supporting information