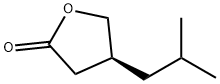
(S)-4-(2-Methyl-1-propyl)-dihydro-2(3H)-furanone synthesis
- Product Name:(S)-4-(2-Methyl-1-propyl)-dihydro-2(3H)-furanone
- CAS Number:172796-26-8
- Molecular formula:C8H14O2
- Molecular Weight:142.2
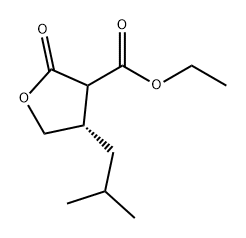
951792-49-7
0 suppliers
inquiry

172796-26-8
3 suppliers
inquiry
Yield:172796-26-8 79.1%
Reaction Conditions:
with water;lithium chloride in dimethyl sulfoxide at 140; for 18 h;
Steps:
3
Example 3 : Preparation of(S) -dihydro-4-isobutylfuran-2 (3H) -one (Compound 3); The compound ((4S) -ethyl tetrahydro-4-isobutyl-2-oxofuran-3-carboxylate, 991 mg, 4.63 mmol) prepared in Example 2 and LiCl (392 mg, 9.25 mmol) were dissolved in DMSO (50 mL) , and then 1 mL of distilled water was added thereto, followed by stirring at 140°Cfor 18 hrs . After the reaction was completed, water was added, and the mixture was extracted with ethyl acetate three times. The organic layer was washed with a saturated ammonium chloride solution and brine once, respectively. The organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by column chromatography using a silica gel (10% ethylacetate/hexane) to obtain Compound 3 ( (S) -dihydro-4-isobutylfuran- 2 (3H) -one) as a colorless oil (520 mg, 79.1%) .The result of H1 NMR (400 MHz, CDCl3) of Compound 3 was as follows: 54.39 (IH, t, J = 8.53 Hz), 3.86 (IH, t, J = 8.74 Hz), 2.60 (2H, m) , 2.13 (IH, m) , 1.55 (IH, m) , 1.34 (2H, t, J = 6.95 Hz), 0.89 (6H, t, J = 6.21 Hz), shown in FIG. 7.The result of 13C NMR (100 MHz, CDCl3) was as follows: 5177.2,
References:
WO2009/22839,2009,A2 Location in patent:Page/Page column 20-21
172796-25-7
1 suppliers
inquiry

172796-26-8
3 suppliers
inquiry
157422-29-2
0 suppliers
inquiry

172796-26-8
3 suppliers
inquiry
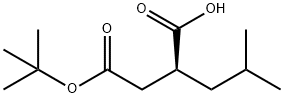
157542-03-5
2 suppliers
inquiry

172796-26-8
3 suppliers
inquiry
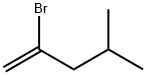
31844-97-0
1 suppliers
inquiry

172796-26-8
3 suppliers
inquiry