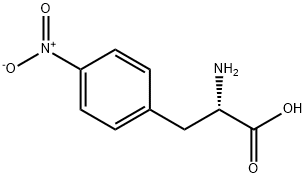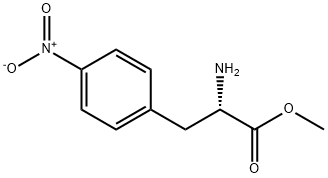
(S)-4-Nitrophenylalaninemethylester synthesis
- Product Name:(S)-4-Nitrophenylalaninemethylester
- CAS Number:81677-60-3
- Molecular formula:C10H12N2O4
- Molecular Weight:224.21
Yield: 98.1%
Reaction Conditions:
with thionyl chloride at 0; for 2 h;Inert atmosphere;Reflux;
Steps:
(S)-Methyl 2-amino-3-(4-nitrophenyl)propanoate (2a)
Thionyl chloride (12 mL) was added dropwise to a solution of 4-nitro-3-phenyl-L-alanine (10 g, 47.58 mmol) in HPLC-grade methanol(150 mL) at 0 C. The mixture was refluxed for 2 h. When thereaction was completed, the reaction mixture was cooled to roomtemperature and removed the solvent under reduced pressure. Theresidue was re-dissolved in water and NaHCO3 was added to adjustthe pH of the solution to 78. The mixture was extracted withDCM for three times. The organic phase was combined, washedwith water and brine. Finally the organic phase was dried withNa2SO4 and concentrated in vacuo to afford the methylated aminoacid as yellow oil (10.46 g, 98.1%). The product was used in thenext step without further purification
References:
Zhu, Biwei;Ge, Jingyan;Yao, Shao Q. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 12,p. 2917 - 2927]
2577-90-4
84 suppliers
$102.00/1g

81677-60-3
11 suppliers
inquiry
636-98-6
18 suppliers
$17.00/5g

81677-60-3
11 suppliers
inquiry