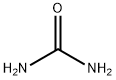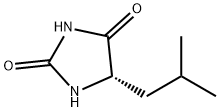
(S)-5-(isobutyl)imidazolidine-2,4-dione synthesis
- Product Name:(S)-5-(isobutyl)imidazolidine-2,4-dione
- CAS Number:40856-75-5
- Molecular formula:C7H12N2O2
- Molecular Weight:156.18
Yield:40856-75-5 72%
Reaction Conditions:
at 170 - 180; for 0.583333 h;
Steps:
2.1.2. Synthesis of thiohydantoins (1a, 1f, and 1n) and hydantoins (2b-g)from direct condensation of L or D-amino acids with urea or thiourea(Adapted from Wang et al., 2006)
General procedure: In a round-bottom flask, the L or D-amino acid (20 mmol) and urea orthiourea (60 mmol) were added and heated on a sand bath at170-180 .C for 35 min under magnetic stirring. The reactions werefollowed by thin-layer chromatography, using ethyl acetate as eluentand potassium permanganate. After consumption of the starting material,the mixture was allowed to cool, and then, 20 mL of distilled icecoldwater was added. The mixture was again heated until the solidsformed during cooling had dissolved, and then cooled in a refrigeratorfor 24 h. The crystallized product formed was filtered and washed with ice-cold distilled water (10 mL). For substances, 2b and 1a, a furtherrecrystallization process from the water were required [11}.
References:
Camargo, Priscila Goes;Fabris, Marciéli;Nakamae, Matheus Yoshimitsu Tatsuta;de Freitas Oliveira, Breno Germano;da Silva Lima, Camilo Henrique;de Fátima, ?ngelo;de Lima Ferreira Bispo, Marcelle;Macedo, Fernando [Chemico-Biological Interactions,2022,vol. 365,art. no. 110045]
7517-19-3
358 suppliers
$5.00/5g

40856-75-5
1 suppliers
inquiry