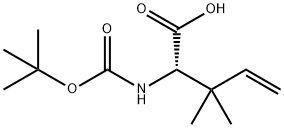
(S)-Boc-2-amino-3,3-dimethyl-pent-4-enoic acid synthesis
- Product Name:(S)-Boc-2-amino-3,3-dimethyl-pent-4-enoic acid
- CAS Number:676629-90-6
- Molecular formula:C12H21NO4
- Molecular Weight:243.3
![4-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-3,3-dimethyl-, methyl ester, (2R)-](/CAS/20210305/GIF/854250-88-7.gif)
854250-88-7
0 suppliers
inquiry

676629-90-6
18 suppliers
$202.50/250mg
Yield:676629-90-6 42%
Reaction Conditions:
with hydrogenchloride;water pH=4;
Steps:
P1'
To a 50-L three- neck flask equipped with a pH electrode, an overhead stirrer a heating mantle and a base addition line, was added the racemic ester 1 (78 g, 0.30 mol) in CH3CN (280 mL). A mixture of Alcalase (350 mL from a 5 x concentrated crude SOLUTION-ALCALASE was passed through the tangential filtration system and concentrated to 1/5 of the original volume before us) and DISTILLED H2O (2.80 L) was then prepared at pH = 7.0. The enzyme solution was added to the reaction flask. The suspension was then stirred at 30 ° C for 51 h. The pH of the solution was maintained at 7.0 by adding 1 N NAOH. Reaction was followed by RP-HPLC looking at both conversion and ee of the product, and stopped after 45 % starting material had been consumed (after 51 h under these conditions, 95.8 mL of 1 N NaOH added). The mixture was extracted MTBE (3 x 1.75 L), and the combined organic layers dried (MGS04) and concentrated under vacuum to afford 50.8 G of crude scalemic ester 3, (R)-enriched (>55 % yield, approx. 56 % ee). This crude mixture contained some carboxylic acid <7 %, which was recovered later by acid-base extraction. The remaining aqueous solution was passed through a Pellicon 2 tangential flow filtration equipped with an ULTRACEL cellulose membrane. During this step most of the enzyme. is removed from the aqueous solution. The remaining solution was acidified to pH 4.0 with concentrated HCI and extracted with MTBE (3 x 1.75 L). The acid fractions were combined and dried (NA2SO4) and concentrated under vacuo. A pale yellow oil acid 2 was obtained (31 G, 91.4 % ee, 42 % yield, >98 % HPLC PURE). HNMR (300 MHz, CDCl3) : 8 10. 69 (s, 1H 5.78 (dd, 2H5.02 (m, 2H4.96 (s, 1H4. 09 (d, 1H1. 36 (s, 9H1. 06 (s, 6HPPMTHE RP-HPLC conditions to detect racemic acid 2: Detector wavelength: 200 nm; Column: Chiralcel OJ-R, 3 um, C-184. 6 x 100 mm; Flow rate 0.5 ml/min ; Injection volume : 10 uL ; Mobil Phases: A: 25 mM NAH2PO4PH 2.0 ; B : Acetonitrile ; Run: Isocratic : 25 % B for 55 min, 3 min post run; Retention times: (R)-ACID. 2-16.33 min and (S) -Acid 2-17.97 min; (S) -Ester 3-50.40 min and (R)-ESTER 3-51. 30 min.
References:
WO2005/26114,2005,A1 Location in patent:Page/Page column 107
![4-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-3,3-dimethyl-](/CAS/20210111/GIF/82706-50-1.gif)
82706-50-1
3 suppliers
inquiry

676629-90-6
18 suppliers
$202.50/250mg
![4-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-3,3-dimethyl-, methyl ester](/CAS/20210305/GIF/192330-57-7.gif)
192330-57-7
0 suppliers
inquiry

676629-90-6
18 suppliers
$202.50/250mg