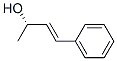
(S,E)-4-Phenyl-3-butene-2-ol synthesis
- Product Name:(S,E)-4-Phenyl-3-butene-2-ol
- CAS Number:81176-43-4
- Molecular formula:
- Molecular Weight:0
Yield:62413-47-2 99%
Reaction Conditions:
with bromopentacarbonylmanganese(I);C35H36FeN3P;potassium tert-butylate;hydrogen in methanol;toluene at 40; under 22502.3 Torr; for 6 h;Catalytic behavior;enantioselective reaction;Pressure;Reagent/catalyst;Solvent;Temperature;
Steps:
7.1-11.2 Preparation of (E)-4-phenylbutyl-3-en-2-ol 2A
(1) Add ligand L3 (6.4mg, 0.011mmol), Mn(CO)5Br (2.7mg, 0.001mmol) into the reaction flask, add toluene (2mL) under argon atmosphere, and stir at 60°C for 0.5h. Prepare a catalyst solution. (2) Under argon atmosphere, add (E)-4-phenylbutyl-3-en-2-one 1A (10mmol), The catalyst solution (0.001 mmol), methanol (20 mL) and potassium tert-butoxide (0.5 mmol) prepared in step (1), After replacing hydrogen, carry out asymmetric hydrogenation reaction at 40°C and 3MPaH2 for 6 hours, The reaction solution was filtered through celite, and the filtrate was concentrated under reduced pressure to recover the solvent to obtain (R,E)-4-phenylbutyl-3-en-2-ol 2A represented by formula (2) with a yield of 99 %, the ee value of the product is 76%.
References:
CN111875474,2020,A Location in patent:Paragraph 0044-0067
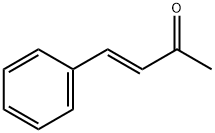
1896-62-4
218 suppliers
$11.00/100g

81176-43-4
2 suppliers
inquiry
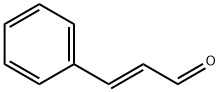
14371-10-9
315 suppliers
$6.00/25g
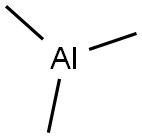
75-24-1
153 suppliers
$54.50/100ml

81176-43-4
2 suppliers
inquiry
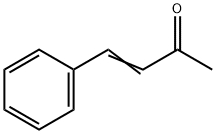
122-57-6
408 suppliers
$5.00/25g

81176-43-4
2 suppliers
inquiry

14371-10-9
315 suppliers
$6.00/25g

544-97-8
129 suppliers
$150.00/5g
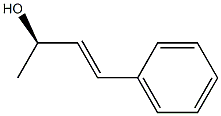
62413-47-2
3 suppliers
inquiry

81176-43-4
2 suppliers
inquiry