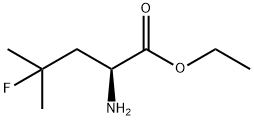
(S)-ethyl 2-aMino-4-fluoro-4-Methylpentanoate synthesis
- Product Name:(S)-ethyl 2-aMino-4-fluoro-4-Methylpentanoate
- CAS Number:156047-39-1
- Molecular formula:C8H16FNO2
- Molecular Weight:177.22
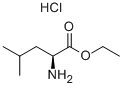
2743-40-0
246 suppliers
$5.00/5g

156047-39-1
45 suppliers
$90.00/5mg
Yield:97 % ee
Reaction Conditions:
with tetrakis(tetrabutylammonium)decatungstate(VI);N-fluorobis(benzenesulfon)imide in water;acetonitrile; for 66 h;Inert atmosphere;Sealed tube;UV-irradiation;Solvent;Concentration;Time;
Steps:
1
[0078] On a larger scale, a solution of (L)-leucine ethyl ester hydrochloride salt (100 mg, 0.51 mmol), TBADT (37 mg, 2%) and NFSI (193 mg, 0.61 mmol) in CH3CN/H20 (2: 1, 6.0 mL) was purged with nitrogen (10 minutes) then sealed. The resulting solution was then placed between two 15 watt UVB (365 nm) lamps and irradiated for 18 hours. At this time, another aliquot of solid NFSI (75 mg) was added to the solution and it was purged with nitrogen for another 10 minutes. The resulting solution was irradiated between two 15 watt UVB (365 nm) lamps for another 24 hours. At this time more solid NFSI (50 mg) was added to the solution and it was purged with nitrogen for another 10 min, and irradiated for a further 24 hours, and then worked up as follows: The blue solution was diluted with CHC13 and water/potassium carbonate was added to pH >10. The solvent was removed via rotary evaporator and the resulting white solid was suspended in CHCI3 and dried over MgSC>4. The solution was cooled in a freezer and then filtered over celite, washing with cold CHCI3. Concentration of the filtrate yielded an orange oil (68 mg) that was -75% pure based on H NMR analysis. The approximate isolated yield of (
References:
WO2015/76,2015,A1 Location in patent:Paragraph 0078
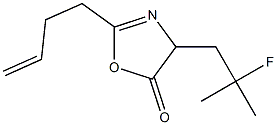
848949-90-6
0 suppliers
inquiry

156047-39-1
45 suppliers
$90.00/5mg
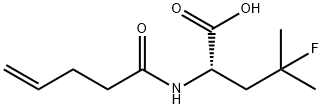
848949-88-2
6 suppliers
inquiry

156047-39-1
45 suppliers
$90.00/5mg

848949-86-0
0 suppliers
inquiry

156047-39-1
45 suppliers
$90.00/5mg
![2-[(Diphenylmethylene)amino]-4-methyl-4-pentenoic acid ethyl ester](/CAS/GIF/80741-44-2.gif)
80741-44-2
26 suppliers
inquiry

156047-39-1
45 suppliers
$90.00/5mg