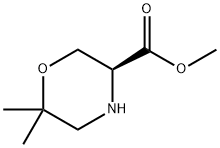
(S)-METHYL 6,6-DIMETHYL-MORPHOLINE-3-CARBOXYLATE synthesis
- Product Name:(S)-METHYL 6,6-DIMETHYL-MORPHOLINE-3-CARBOXYLATE
- CAS Number:947729-86-4
- Molecular formula:C8H15NO3
- Molecular Weight:173.21
1001054-45-0
2 suppliers
inquiry

947729-86-4
28 suppliers
$200.00/25mg
Yield:-
Reaction Conditions:
with hydrogen;palladium(II) hydroxide/carbon in methanol;acetic acid; under 2585.81 Torr; for 35 h;
Steps:
3.2
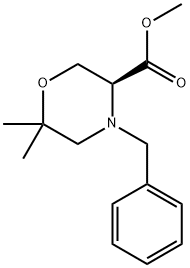
Step 2. (S)-Methyl 6,6-dimethylmorpholine-3-carboxylate. The mixture of diastereomers obtained in step 1 is combined with tributyltin hydride (73 mL, 272 mmol) in 400 mL refhixing toluene. AIBN (1 g, 6 mmol) dissolved in 40 mL toluene is added dropwise. After 2 h reflux, the reaction mixture is cooled to it. Saturated aqueous KF solution (800 mL) is added and the resulting heterogeneous mixture is filtered through Celite and the sod washed with EtOAc. The combined solutions are transferred to a separatory funnel and washed twice with 200 mL saturated KF, and then once with 20 mL brine. The organic phase is dried over MgSO4, filtered and concentrated in vacuo. The product is purified by flash chromatography eluting with 95/5 hexanes/EtOAc followed by EtOAc to afford (S)-methyl 4-benzyl-6,6-dimethylmorphoIine-3- carboxylate as a clear oil. This oil is dissolved in a mixture of 200 mL MeOH and 20 mL acetic acid. Palladium hydroxide (5 g, 20 wt. % on carbon) is added and the reaction mixture hydrogenated on a Paar apparatus at 50 psi for 10 h. An additional 5 g of palladium hydroxide is added and the hydrogenation is continued at 50 psi for another 25 h. The reaction mixture is filtered through Celite and concentrated in vacuo to afford the title compound. 1H NMR (400 MHz, d-6-DMSO) δ: 3.71-3.74 (m, IH), 3.61-3.65 (m, IH), 3.61 (s, 3H), 3.36 (m, IH), 2.65 (d, IH), 2.46 (d, IH), 1.1 1 (s, 3H)5 1.09 (s, 3H).
References:
WO2008/24692,2008,A1 Location in patent:Page/Page column 42