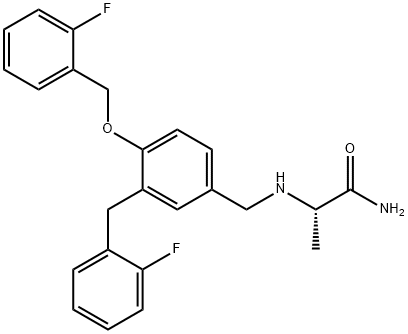
Safinamide Impurity 9 synthesis
- Product Name:Safinamide Impurity 9
- CAS Number:1000370-28-4
- Molecular formula:C24H24F2N2O2
- Molecular Weight:410.46
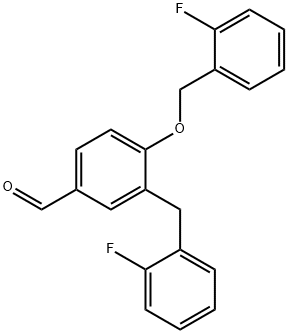
1000370-25-1
11 suppliers
inquiry
33208-99-0
375 suppliers
$6.00/5g

1000370-28-4
20 suppliers
inquiry
Yield:1000370-28-4 83%
Reaction Conditions:
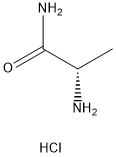
Stage #1: 3-(2-fluoro-benzyl)-4-(2-fluoro-benzyloxy)-benzaldehyde;(S)-alaninamide hydrochloridewith triethylamine in methanol at 20; for 1 h;
Stage #2: with hydrogen;platinum on carbon in methanol;water at 35; under 3750.38 Torr; for 3 - 5 h;
Steps:
8.b
To 3-(2-fluorobenzyl)-4-(2-fluorobenzyloxy)benzaldehyde (3.56 g, 0.0105 mol) in a 50 mL flask, a solution previously prepared by cautiously adding under stirring triethylamine (1.2 g, 0.01 19 mol) to a 17 mL methanol solution of L-alaninamide hydrochloride (1.48 g, 0.01 19 mol), is added at room temperature. This reaction mixture is stirred for 1 hour at room temperature
References:
WO2009/74478,2009,A1 Location in patent:Page/Page column 51-52