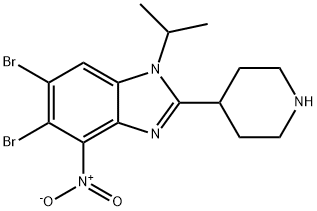
SEL24-B489 synthesis
- Product Name:SEL24-B489
- CAS Number:1616359-00-2
- Molecular formula:C15H18Br2N4O2
- Molecular Weight:446.14
Yield: 1.9 g
Reaction Conditions:
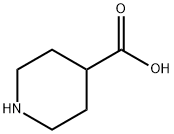
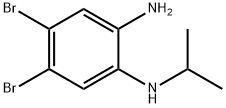
Stage #1:isonipecotic acid;4,5-dibromo-1-N-(propan-2-yl)benzene-1,2-diamine with phosphoric acid at 180; for 3.5 h;
Stage #2: with sulfuric acid;potassium nitrate at 0 - 20;
Steps:
26A 3.9. Compounds of Example 26: 5 ,6-dibromo-4-nitro-2-(piperidin-4-yl)- 1 -(propan-2-yl)- 1 H- 1 ,3-benzodiazole (Example 26 A)
3.9. Compounds of Example 26: 5 ,6-dibromo-4-nitro-2-(piperidin-4-yl)- 1 -(propan-2-yl)- 1 H- 1 ,3-benzodiazole (Example 26 A): 4,5-dibromo-l-N-(propan-2-yl)benzene-l,2-diamine (2,8g, 9,lmmol) and isonipeconic acid (l,17g, 9,lmmol) were taken up in phosphoric acid (17,82g, 0,18mol). The resulting mixture was stirred at 180°C for 3,5 hours. The mixture was allowed to cool to RT and diluted with water to 200ml. The solution was basified to pH 14.0 using solid NaOH. The resulting precipitate was then filtered off and washed repeatedly with MeOH. The filtrate was concentrated in-vacuo. The product was purified on A1203 (basic) using DCM/MeOH/NH3 sat. in MEOH (25: 15: 1). The obtained product (8,7mmol, 3,9g) was dissolved in cone. H2S04 (30ml). Next KN03 (8,7mmol, 0,89g) was added in one portion at 0° C. The resulting mixture was stirred at 0°C for 3h and at RT overnight. Then the mixture was poured onto ice. The product was filtered and washed with water.The product was purified on on A1203 (basic) using DCM/MeOH/NH3 sat. in MEOH (25: 15: 1) to afford 5,6-dibromo-4- nitro-2-(piperidin-4-yl)-l-(propan-2-yl)-lH-l,3-benzodiazole (l,9g). 1H NMR (600 MHz, DMSO) δ 8.74 (bs, 1H), 8.48 (s, 1H), 8.35 (bs, 1H), 4.94 (hept, J = 6.8 Hz, 1H), 3.52 - 3.46 (m, 1H), 3.42 - 3.37 (m, 2H), 3.08 (bs, 2H), 2.07 - 1.96 (m, 4H), 1.60 (d, J = 6.9 Hz, 6H). m/z 446,8; rt 2,7min.
References:
SELVITA S.A.;CZARDYBON, Wojciech;BRZÓZKA, Krzysztof;GALEZOWSKI, Michal;WINDAK, Renata;MILIK, Mariusz;ZAWADZKA, Magdalena;GUZIK, Pawel;WINCZA, Ewelina;PROKOP, Marta;WIKLIK, Katarzyna;SABINIARZ, Aleksandra;CHOLODY, Wieslaw Marek;HORVATH, Raymond;RZYMSKI, Tomasz WO2014/96388, 2014, A2 Location in patent:Page/Page column 121-122
89365-48-0
7 suppliers
inquiry

1616359-00-2
4 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1616359-00-2
4 suppliers
inquiry