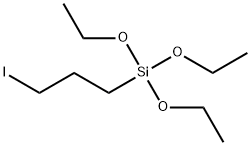
Silane, triethoxy(3-iodopropyl)- synthesis
- Product Name:Silane, triethoxy(3-iodopropyl)-
- CAS Number:57483-09-7
- Molecular formula:C9 H21 I O3 Si
- Molecular Weight:332.25
5089-70-3
317 suppliers
$10.00/5g

57483-09-7
6 suppliers
inquiry
Yield:57483-09-7 95%
Reaction Conditions:
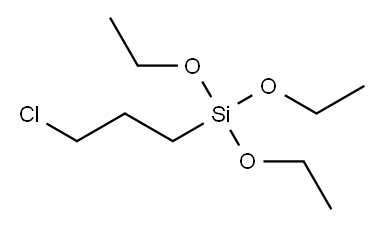
with sodium iodide in propan-2-one at 60; for 60 h;Inert atmosphere;Schlenk technique;
Steps:
2.1 4.2.1. 3-Iodopropyl-triethoxysilane (IPTES)
To a stirred solution of NaI (6.00 g, 40 mmol) in anhydrous acetone (40 mL) in a 250 mL three-neck round bottom flask equipped with a condenser and a pressure-equalizing dropping funnel, 3 (chloropropyl)triethoxysilane (CPTES) (4.82 g, 20 mmol) was added dropwise. The reaction mixture was stirred at 60 °C for 60 h under N2 and then cooled to room temperature. The solvent was removed under reduced pressure and the solid residue was washed with diethyl ether three times. The combined filtrate was concentrated under reduced pressure. The crude product was purified by column chromatography using hexane-ethyl acetate (10:1) as the eluent to afford the pure 3-iodopropyl-triethoxysilane (IPTES) as a colorless oil (6.31 g, 95%). 1H NMR (400 MHz, d6-DMSO): δ 0.64-0.68 (m, 2H, CH2Si), 1.15 (t, 9H, J = 6.0 Hz, CH3), 1.75-1.83 (m, 2H, CH2), 3.28 (t, 2H, J = 6.0 Hz, ICH2), 3.75 (q, 6H, J = 8.0 Hz, OCH2). 13C{1H} NMR (100 MHz, d6-DMSO): δ 11.6 (CH2Si), 12.4 (CH2), 18.2 (CH3), 27.3 (OCH2), 57.8 (SiOCH2).
References:
He, Dongmei;Horváth, István T. [Journal of Organometallic Chemistry,2017,vol. 847,p. 263 - 269]