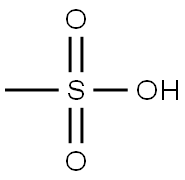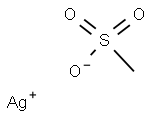
Silver mesylate synthesis
- Product Name:Silver mesylate
- CAS Number:2386-52-9
- Molecular formula:CH3AgO3S
- Molecular Weight:202.97
Yield:2386-52-9 99%
Reaction Conditions:
in water at 90; for 3 h;
Steps:
I.f f) Comparative compound 2 (CC2 )
To a solution of methanesulfonic acid (77.7 g, 800 mmol, 1 .0 eq) in H2O (1000 ml) was added silver oxide (93.3 g, 398 mmol, 0.5 eq) at room temperature, and the mixture was stirred at 90 0 C for 3 h. Dark black suspension was obtained. The solvent of the reaction mixture was reduced under the reduced pressure and the precipitation was removed by filtration. Acetone was added to the mother liquid and precipitations are washed by acetone to give a white solid. The obtained silver metylsulfonic acid salt was dried over reduced pressure and used further purification (161 g, 99% yield). To a solution of silver metylsulfonic acid salt (161 g, 790 mmol, 1.0 eq) in acetonitrile (800 ml) was added iodoacetic acid (148 g, 790 mmol, 1.0 eq) at room temperature, and the mixture was stirred for 15 h. The solvent of the reaction mixture was reduced under the reduced pressure. AcOEt was added and the obtained suspension was filtrated. The mother solution was concentrated again under the reduced pressure. The solid was dissolved by EtOAc and hexane was added to make a suspension. The suspension was filtrated and washed by EtOAc/Hexane to give a white solid. The obtained mesyl-glycolic acid was dried over reduced pressure and used further purification (57.8 g, 47% yield). To a suspension of mesyl-glycolic acid (28.3 g, 180 mmol, 1 .0 eq), propargyl alcohol (30.6 g, 540 mmol, 3.0 eq) and 4-(dimethylamino)-pyridine (DMAP, 2.22, 18 mmol, 0.1 eq) in CH2CI2 (1000 ml) was added 1 -(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI , 42.3 g, 216 mmol, 1.2 eq) at ice bath temperature, and the mixture was stirred at room tempera- ture for 15 h. The reaction was quenched with sat NH4CI aq solution, extracted with water, washed with bine, and dried over anhydrous Na2S04. The solvent was removed under reduced pressure and the crude product was purified by silica gel chromatography (hexanes/EtOAc) to give the product. The obtained oil was purified again by distillation to give the desired molecule as a color less oil (19.5 g, 56% yield).
References:
BASF SE;YOSHIDA, Kazuki;FUKUZUMI, Takeo;KIM, Jinbum;SAWADA, Eri;SCHULZ-DOBRICK, Martin WO2018/134251, 2018, A1 Location in patent:Page/Page column 26-27