
SodiuM 2,4,6-triMethylbenzenesulfinate synthesis
- Product Name:SodiuM 2,4,6-triMethylbenzenesulfinate
- CAS Number:50827-54-8
- Molecular formula:C9H13NaO2S
- Molecular Weight:208.25
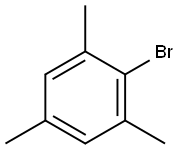
576-83-0
247 suppliers
$31.00/25g

50827-54-8
17 suppliers
$220.00/2.5g
Yield:50827-54-8 69%
Reaction Conditions:
Stage #1: 2,4,6-trimethylphenyl bromidewith iodine;magnesium in tetrahydrofuran at 100; for 1 h;Inert atmosphere;Microwave irradiation;
Stage #2: with 1,4-diazabicyclo [2.2.2] octane-1,4-diium-1,4-disulfinate in tetrahydrofuran at 0; for 3 h;Inert atmosphere;
Stage #3: with sodium carbonate in tetrahydrofuran;diethyl ether;water;Reagent/catalyst;Temperature;
Steps:
General procedure, Synthesis of sodium arylsulfinate by Grignard reagent
General procedure: The aryl bromide (2.00 mmol), Mg (194 mg, 8.00 mmol) and an I2 crystal were weighed in a 2-5 ml Smith process vial and 2.5 ml dry THF was added after flushing with nitrogen. The mixture was heated in a microwave reactor for 1 h at 100 °C. In a second vial DABSO (121 mg, 0.500 mmol) was weighed and cooled on ice followed by slow addition of the Grignard reagent by syringe and the mixture was then stirred for 3 hours at 0 °C and then allowed to reach room temperature. The reaction mixture was poured into 10 ml of diethyl ether and was extracted with 3×10 ml of 10% Na2CO3 solution. The aqueous layers were acidified by careful addition of 3 ml of concentrated H2SO4while cooling on ice and was then extracted with 3×10 ml of diethyl ether. The organic layers were then extracted with 3×10 mlof 10% Na2CO3 solution. The water was evaporated in vacuo. 20 ml of absolute ethanol was added to the residue and refluxed for 10 minutes. The round bottomed flask was allowed to cool and was then filtered through a P4 frit, and the ethanol extraction was repeated. The ethanol was evaporated in vacuoand the product was obtained as a white solid.
References:
Skillinghaug, Bobo;Rydfjord, Jonas;Odell, Luke R. [Tetrahedron Letters,2016,vol. 57,# 5,p. 533 - 536] Location in patent:supporting information

773-64-8
261 suppliers
$5.00/5g

50827-54-8
17 suppliers
$220.00/2.5g
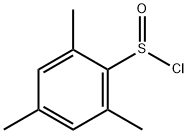
73766-65-1
0 suppliers
inquiry

50827-54-8
17 suppliers
$220.00/2.5g
![Thiophene,2-[(2,4,6-trimethylphenyl)sulfonyl]-](/CAS/20180527/GIF/21991-08-2.gif)
21991-08-2
0 suppliers
inquiry

50827-54-8
17 suppliers
$220.00/2.5g