SODIUM 2-[(TRIFLUOROACETYL)AMINO]BENZOATE synthesis
- Product Name:SODIUM 2-[(TRIFLUOROACETYL)AMINO]BENZOATE
- CAS Number:19165-29-8
- Molecular formula:C9H6F3NO3
- Molecular Weight:233.14
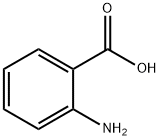
118-92-3
4 suppliers
$23.00/250g
![SODIUM 2-[(TRIFLUOROACETYL)AMINO]BENZOATE](/CAS/GIF/19165-29-8.gif)
19165-29-8
12 suppliers
$63.00/200μmol
Yield:-
Reaction Conditions:
with trifluoroacetic anhydride
Steps:
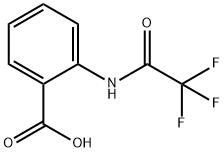
Synthesis of 2-(2,2,2-trifluoroacetamido)benzoic acid (COOH-NHCOCF3, Compound VI)
Synthesis of 2-(2,2,2-trifluoroacetamido)benzoic acid (COOH-NHCOCF3, Compound VI)
To a stirred solution of anthranilic acid (1.87 g, 13.6 mmol) in THF (30 mL), trifluoroacetic anhydride (2.4 mL, 17.3 mmol) was added at 0° C. and the resulting mixture was then stirred at room temperature for 16 h.
Until the end of the reaction, the solvent was concentrated under reduced pressure to give a white powder.
The residue was dissolved in ethyl acetate, washed with saturated NaCl aqueous solution, and dried over anhydrous MgSO4.
The dried organic solution was filtered and concentrated under reduced pressure.
The residue was purified by column chromatography on silica gel (hexane:EtOAc=5:2) to afford COOH-NHCOCF3 (compound VI) (1.85 g, 58%) as white solid. 1H NMR (400 MHz, DMSO-d6, 298 K) δ (ppm): 12.50 (brs, 1H), 8.27 (d, J=8.0 Hz, 1H), 8.05 (d, J=8.0 Hz, 1H), 7.71 (m, 1H), 7.36 (t, J=8.0 Hz, 1H); 13 C NMR (100 MHz, DMSO-d6, 298 K) δ (ppm): 169.3, 154.8, 154.4, 154.1, 153.7, 137.5, 134.3, 131.3, 125.5, 121.3, 119.9, 119.3, 117.0, 114.2, 111.3; 19 F NMR (376 MHz, DMSO-d6, 298 K) δ (ppm): -75.67.
References:
US11508566,2022,B2

118-92-3
4 suppliers
$23.00/250g
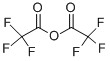
407-25-0
439 suppliers
$9.00/1g
![SODIUM 2-[(TRIFLUOROACETYL)AMINO]BENZOATE](/CAS/GIF/19165-29-8.gif)
19165-29-8
12 suppliers
$63.00/200μmol
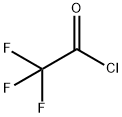
354-32-5
112 suppliers
$55.00/10g

118-92-3
4 suppliers
$23.00/250g
![SODIUM 2-[(TRIFLUOROACETYL)AMINO]BENZOATE](/CAS/GIF/19165-29-8.gif)
19165-29-8
12 suppliers
$63.00/200μmol