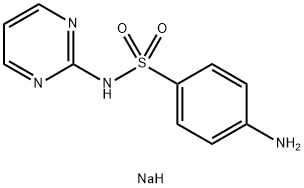
Sodium sulfadiazine synthesis
- Product Name:Sodium sulfadiazine
- CAS Number:547-32-0
- Molecular formula:C10H11N4NaO2S
- Molecular Weight:274.27
68-35-9
446 suppliers
$5.00/100mg

547-32-0
306 suppliers
$5.00/100mg
Yield:-
Reaction Conditions:
with sodium hydroxide in ethanol;waterHeating;
Steps:
2.1. Synthesis and crystallization
The simple salt forms were prepared by reacting 1:1 molarmixtures of sulfadiazine and MOH (M = Li, Na or K) or theorganic base in water-ethanol (50:50 v/v). The mixtures werestirred and heated to give clear solutions, before being left tocool to room temperature. Partial evaporation of these reactionmixtures over periods of 4-7 d gave suitable crystals of(I), (III), (V) and (VI), but a fine powder of Na salt (II).Good-quality crystals of (II) were obtained by vapour diffusionof ethanol into an aqueous solution of sodium sulfadiazine.Na Orange G (OG) complex (IV) was obtained bydissolving NaOG (0.20 g, 0.44 mmol) in the minimum amountof water. A slight excess of sulfadiazine (0.12 g, 0.48 mmol)was also dissolved in the minimum amount of water. The twosolutions were mixed together with stirring and acidified withconcentrated HCl. After 3 d, orange crystals of (IV) hadgrown.
References:
Campbell, Gemma;Fisher, Rebecca;Kennedy, Alan R.;King, Nathan L. C.;Spiteri, Rebecca [Acta Crystallographica Section C: Structural Chemistry,2018,vol. 74,# 4,p. 472 - 479]