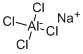
SODIUM TETRACHLOROALUMINATE synthesis
- Product Name:SODIUM TETRACHLOROALUMINATE
- CAS Number:7784-16-9
- Molecular formula:AlCl4Na
- Molecular Weight:191.78
NaCl + A1Cl3 = NaAlCl4
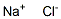
7647-14-5
1124 suppliers
$5.00/500g
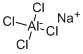
7784-16-9
69 suppliers
$60.00/500mg
Yield:-
Reaction Conditions:
with aluminium trichloride
References:
Korshunov, B. G.;Lapkina, E. D. [Zhurnal Neorganicheskoi Khimii,1963,vol. 8,p. 1354 - 1356][Zhurnal Neorganicheskoi Khimii,1963,vol. 8,p. 2585 - 2588] [Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Na: SVol.6, 6.2.5.9, page 396 - 397]
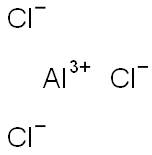
7446-70-0
687 suppliers
$15.00/25g
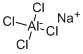
7784-16-9
69 suppliers
$60.00/500mg

7446-70-0
687 suppliers
$15.00/25g
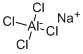
7784-16-9
69 suppliers
$60.00/500mg
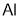
7429-90-5
8 suppliers
$9.00/5g

7446-70-0
687 suppliers
$15.00/25g
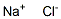
7647-14-5
1124 suppliers
$5.00/500g
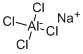
7784-16-9
69 suppliers
$60.00/500mg
7429-90-5
8 suppliers
$9.00/5g
7647-14-5
1124 suppliers
$5.00/500g
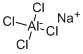
7784-16-9
69 suppliers
$60.00/500mg