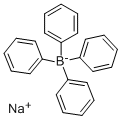
Sodium tetraphenylboron synthesis
- Product Name:Sodium tetraphenylboron
- CAS Number:143-66-8
- Molecular formula:C24H20BNa
- Molecular Weight:342.22
Yield:-
Reaction Conditions:
with magnesium in tetrahydrofuran at 0 - 20; for 12 h;Inert atmosphere;
Steps:
Sodium Tetra-4-tolylborate; Typical Procedure for the Synthesis ofSodium Tetraarylborates
General procedure: Sodium tetraarylborates were prepared according to the literature, with some modifications. NaBF4 (1.0 g, 9.11 mmol) and Mg turnings(0.99 g, 41.00 mmol) were taken in an oven-dried sealed tube. The tube was filled with argon by the standard Schlenk technique. To this mixture, THF (20 mL) and 1,2-dibromoethane (40 μL, 0.46 mmol) were added, and the resulting mixture was stirred for 1 min. Finally,4-bromotoluene (4.7 mL, 38.3 mmol) was added in one shot at 0 °C. A very exothermic reaction started immediately after the addition of aryl halide. After the apparent exotherm had ceased, the resulting suspension was stirred at r.t. for 12 h. The solution became gray and white precipitates were formed. The reaction mixture was then added to aq Na2CO3. The resulting mixture was stirred for 30 min and then extracted with MeCN (3 × 30 mL). The combined organic layerwas washed with brine (50 mL), dried over MgSO4, filtered and concentratedunder reduced pressure with a rotary evaporator. The crude residue was purified by precipitation using Et2O-hexane (1:2) to provide sodium tetra-4-tolylborate as a white powder; yield: 3.48 g (8.75mmol, 96%).
References:
Osuka, Atsuhiro;Vasu, Dhananjayan;Yorimitsu, Hideki [Synthesis,2015,vol. 47,# 21,art. no. 47,p. 3286 - 3291]
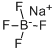
13755-29-8
457 suppliers
$6.00/25g
100-58-3
185 suppliers
$18.00/10ml

143-66-8
433 suppliers
$7.00/5g

3677-81-4
5 suppliers
inquiry

143-66-8
433 suppliers
$7.00/5g
108-86-1
497 suppliers
$10.00/5g

143-66-8
433 suppliers
$7.00/5g
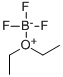
109-63-7
382 suppliers
$10.60/1gm:
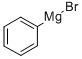
100-58-3
185 suppliers
$18.00/10ml

143-66-8
433 suppliers
$7.00/5g