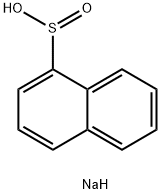
SODIUMNAPHTHIONATE synthesis
- Product Name:SODIUMNAPHTHIONATE
- CAS Number:64326-13-2
- Molecular formula:C10H9NaO2S
- Molecular Weight:216.23
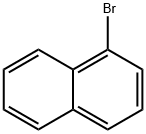
90-11-9
517 suppliers
$5.00/10g

64326-13-2
22 suppliers
inquiry
Yield:64326-13-2 82%
Reaction Conditions:
Stage #1: 1-Bromonaphthalenewith n-butyllithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2: with 1,4-diazabicyclo [2.2.2] octane-1,4-diium-1,4-disulfinate in tetrahydrofuran at -78; for 3 h;Inert atmosphere;
Stage #3: with sodium carbonate in tetrahydrofuran;diethyl ether;water;Reagent/catalyst;Temperature;
Steps:
General procedure, Synthesis of sodium arylsulfinate by aryl lithium reagent
General procedure: The aryl bromide (2.00 mmol) was weighed in a 2-5 ml Smith process vial and 2.5 ml dry THF was added after flushing with nitrogen. The mixture was cooled to -78 °C in a dry ice/acetone bath and n-BuLi (0.80 ml, 2.5 M, 2.0 mmol) was added by syringe. In a second vial DABSO (121 mg, 0.500 mmol) was weighed and cooled in a dry ice/acetone bath followed by slow addition of the aryl lithium reagent by syringe and the mixture was then stirred for 3 hours at -78 °C and then allowed to reach room temperature. In cases where the lithium reagent was insoluble at -78 °C, the vial was allowed to warm until the reagent became sufficiently soluble before addition. The reaction mixture was worked up and purified as described above.
References:
Skillinghaug, Bobo;Rydfjord, Jonas;Odell, Luke R. [Tetrahedron Letters,2016,vol. 57,# 5,p. 533 - 536] Location in patent:supporting information
85-46-1
185 suppliers
$19.00/1g

64326-13-2
22 suppliers
inquiry
529-36-2
84 suppliers
$55.22/1g

64326-13-2
22 suppliers
inquiry
90-14-2
290 suppliers
$19.00/5g

64326-13-2
22 suppliers
inquiry