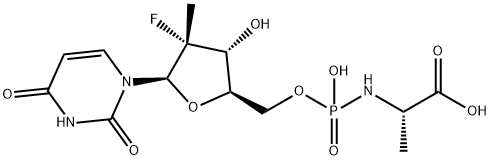
Sofosbuvir metabolites GS566500 synthesis
- Product Name:Sofosbuvir metabolites GS566500
- CAS Number:1233335-78-8
- Molecular formula:C13H19FN3O9P
- Molecular Weight:411.28
1064684-44-1
18 suppliers
inquiry

1233335-78-8
30 suppliers
inquiry
Yield:1233335-78-8 92%
Reaction Conditions:
with water;triethylamine at 60; for 30 h;
Steps:
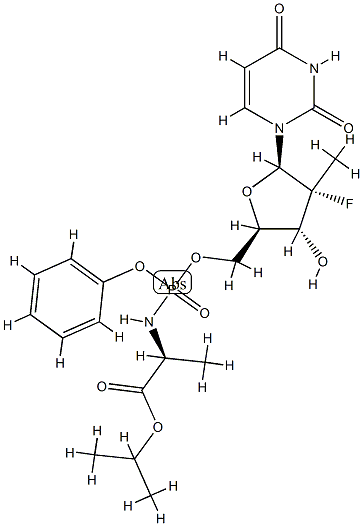
Compound 16 ((2S)-isopropyl 2-((((2R,3R,4R,5R)-5-(2,4-dioxo-3,4- dihydropyrimidin- 1 (2H)-yl)-4-fluoro-3 -hydroxy-4-methyltefrahydrofuran-2- yl)methoxy)(phenoxy)phosphoryl-amino)propanoate) was prepared according to the procedures disclosed at pages 652-669 of U.S. Patent Application No. 12/053,015 (Attorney Docket No. 60137.0034USU1) filed March 21, 2008 the identified subject matter of which is incorporated by reference in its entirety. (Compound 16 is identified as compound 25 at page 674 of 12/053,015.)Compound 17 was synthesis from compound 15 by the following procedure. Compound 16 (300 mg, 0.57 mmol) was suspended in triethylamine (6 mL) and water (1.5 mL), and heated at 60°C for 30 h. The volatile components were then evaporated under reduced pressure. The crude product was purified by flash column chromatography on silica gel using 50-70% isopropyl alcohol in dichloromethane followed by 0-20% ammonium hydroxide in isopropyl alcohol as eluents. The product (2) was obtained as a white solid (210 mg, 92% yield): 1H NMR (400 MHz, DMSO-d6) δ 8.01 (d, J = 8.0 Hz, IH), 5.97 (d, J = 18.8 Hz, IH), 5.55 (d, J = 8.0 Hz, IH), 4.02-3.80 (m, 4H), 3.34 (dq, J = 7.2, 10.4 Hz, IH), 1.25 (d, J = 22.4 Hz, 3H), 1.10 (d, J = 6.8 Hz, 3H); 31P NMR (162 MHz, DMSO-d6) δ 7.91; MS (ESI) (M + H)+ 412.3.
References:
WO2010/75517,2010,A2 Location in patent:Page/Page column 32-33