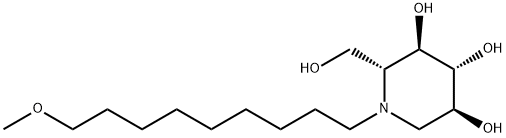
SP187 synthesis
- Product Name:SP187
- CAS Number:615253-61-7
- Molecular formula:C16H33NO5
- Molecular Weight:319.44
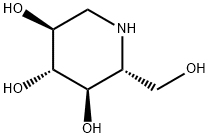
19130-96-2
368 suppliers
$40.00/20mg
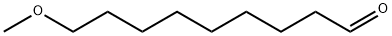
58257-19-5
5 suppliers
inquiry

615253-61-7
19 suppliers
inquiry
Yield:615253-61-7 28.1%
Reaction Conditions:
Stage #1: 1,5-dideoxy-1,5-imino-D-glucitol;9-methoxy-1-nonanal in ethanol at 20;Inert atmosphere;
Stage #2: with hydrogen;palladium on carbon in ethanol at 20;
Steps:
3.3c
3 c Synthesis of N-(9-methoxy)-nonyl DNJ Table 7. Materials for synthesis of N-(9-methoxy)-nonyl DNJProcedure: a 50-mL, two-necked, round-bottom flask equipped with magnetic stirrer and a stir bar was charged with DNJ (300 mg, 1.84 mmol), ethanol (20 mL), 9-methoxy- 1 -nonanal (476 mg, 2.76 mmol) at room temperature. The reaction mixture was stirred for 5-10 minutes under nitrogen and Pd/C was added at room temperature. The reaction mixture was evacuated and was replaced by hydrogen gas using a balloon. This process was repeated three times and then reaction mixture was stirred under atmospheric hydrogen at room temperature. The progress of reaction was monitored by TLC (Note 1). The reaction mixture was filtered through a bed of Celite and was washed with ethanol (20 mL). The filtrate was concentrated in vacuo to get a crude product. The crude product was purified by column chromatography using 250-400 mesh silica gel (20 g). A solvent gradient of methanol in ethyl acetate (5- 25%) was used to elute the product from the column. All fractions containing the desired pure product were combined, and concentrated in vacuo to give an off white solid. The solid was triturated in ethyl acetate (20 mL), filtered and dried in high vacuum to give a white solid [lot: D-1027-158 (165.3 mg, 28.1%). Completion of the reaction was monitored by thin layer chromatography (TLC) using a thin layer silica gel plate; eluent: 50% methanol indichloromethane .
References:
WO2011/28781,2011,A1 Location in patent:Page/Page column 16