
Spermidine synthesis
- Product Name:Spermidine
- CAS Number:124-20-9
- Molecular formula:C7H19N3
- Molecular Weight:145.25

Synthesis and Characterization of a Linker for Primary Amines used in the Solid Phase Organic Synthesis of Spermidine
J. Braz. Chem. Soc., Vol. 22, No. 1, 86-91, 2011
DOI: 10.1590/S0103-50532011000100011
![Carbamic acid, N-[4-[(3-aminopropyl)amino]butyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/103493-34-1.gif)
103493-34-1
0 suppliers
inquiry

124-20-9
360 suppliers
$12.00/250mg
Yield:124-20-9 79.5%
Reaction Conditions:
with hydrogenchloride in methanol; for 4 h;Cooling with ice;
Steps:
1.S3; 2.S3; 3.S3; 4.S3; 5.S3
dissolving the compound d (0.3 mol) in the previous step in 450 mL of methanol, adding hydrochloric acid (250 mL, 3 mol) dropwise to an ice-water bath, stirring for 4 h, removing methanol under reduced pressure, and adjusting with 40% aqueous sodium hydroxide solution to pH=12, de-dried under reduced pressure, add 300 mL of tetrahydrofuran for extraction, filter, concentrate the filtrate to dryness, further rectify under reduced pressure, collect fractions at 124-126 °C under 12 mmHg vacuum to obtain compound e, namely spermidine 34.6 g, yield 79.5%, gas phase purity 99.5%.
References:
CN113735716,2021,A Location in patent:Paragraph 0032-0037
![Carbamic acid, [4-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)butyl][3-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)propyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210111/GIF/177085-34-6.gif)
177085-34-6
0 suppliers
inquiry

124-20-9
360 suppliers
$12.00/250mg
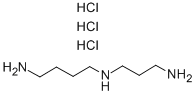
334-50-9
268 suppliers
$23.30/1G

124-20-9
360 suppliers
$12.00/250mg
![1,3,5-Triazin-2(1H)-one, tetrahydro-1,3-bis(phenylmethyl)-5-[3-[[4-[tetrahydro-4-oxo-3,5-bis(phenylmethyl)-1,3,5-triazin-1(2H)-yl]butyl]amino]propyl]-](/CAS/20210305/GIF/143565-69-9.gif)
143565-69-9
0 suppliers
inquiry

124-20-9
360 suppliers
$12.00/250mg
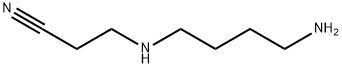
4748-73-6
11 suppliers
inquiry

124-20-9
360 suppliers
$12.00/250mg