SPIRO[2.4]HEPTA-4,6-DIENE synthesis
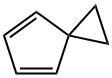
- Product Name:SPIRO[2.4]HEPTA-4,6-DIENE
- CAS Number:765-46-8
- Molecular formula:C7H8
- Molecular Weight:92.14
Yield:765-46-8 70%
Reaction Conditions:
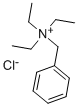
with 2,6-di-tert-butyl-4-methyl-phenol;N-benzyl-N,N,N-triethylammonium chloride;sodium hydroxide in water at 30 - 40; for 1.5 h;
Steps:
1.2 Preparation of spiro[2.4]hepta-4,6-diene
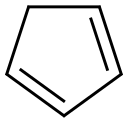
The synthesis of spiro[2.4]hepta-4,6-diene (1) was carried out according to a modifiedmethod [2]. A mixture of freshly distilled cyclopentadiene (9.2 ml, 7.2 g, 0.11 mol), 1,2-dichloroethane (8.6 ml, 10.8 g, 0.11 mol) 2,6-di-tert-butyl-4-methylphenol (20 , 0.1 mmol)was added dropwise over 30 min to a vigorously stirred mixture of NaOH (50% aqueous solution, 24 g, 0.6 mol) and triethylbenzylammonium chloride (TEBA) (0.2 g, 1 mmol) at 30-40°C. The reaction mixture was stirred for 1 h at the same temperature. The reaction mixture wasdistilled with steam, then the organic layer was distilled under reduced pressure (60-70°/150-200 mmHg). The product yield (1) was 7.0 g (70%), purity 99% (by GLC). Physical and spectroscopic data were consistent with the literature data [2].
References:
Shulishov, Evgeny V.;Pantyukh, Olga A.;Menchikov, Leonid G.;Tomilov, Yury V. [Tetrahedron Letters,2019,vol. 60,# 31,p. 2043 - 2045] Location in patent:supporting information
4984-82-1
117 suppliers
$76.50/0.1mole

106-93-4
376 suppliers
$15.00/5g
![SPIRO[2.4]HEPTA-4,6-DIENE](/CAS/GIF/765-46-8.gif)
765-46-8
36 suppliers
$119.00/1ml
![Bicyclo[3.2.0]hept-2-ene](/CAS/20200611/GIF/7095-65-0.gif)
7095-65-0
0 suppliers
inquiry
![SPIRO[2.4]HEPTA-4,6-DIENE](/CAS/GIF/765-46-8.gif)
765-46-8
36 suppliers
$119.00/1ml