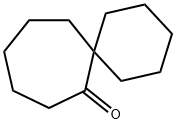
Spiro[5.6]dodecan-7-one synthesis
- Product Name:Spiro[5.6]dodecan-7-one
- CAS Number:4728-90-9
- Molecular formula:C12H20O
- Molecular Weight:180.29
Yield:4728-90-9 77%
Reaction Conditions:
with sodium hydride in tetrahydrofuran; for 10 h;Reflux;
Steps:
General Procedure for the Preparation of SpirocyclicKetones 3-6
General procedure: To a magnetically stirred suspension of sodium hydride(3 mol equivalents) in 100 mL refluxing anhydrous THF wasadded a mixture of six-/seven-membered cycloalkanone (10g) and 1,4-dibromobutane or 1,5-dibromopentane (1.2 molequivalents) over a period of 40 min. After completion ofaddition, the mixture was further refluxed for 10 hrs. Monitoringthe reaction using GC indicated complete conversionof starting cycloalkanone. The reaction mixture was cooled,diluted with 50 mL ether and added to 100 mL of ice coldwater. The organic layer was separated, and the aqueouslayer extracted with ether (3 x 50 mL). The combined etherealextracts were washed with water (2 x 50 mL), brine (2 x30 mL) and dried over anhydrous potassium carbonate. Thesolvent was removed on a rotary evaporator and distilledunder reduced pressure to isolate the spirocyclic ketones 3-6.
References:
Venkatesha, Manjunatha Achanna;Suresh, HariPrasad [Letters in Organic Chemistry,2013,vol. 10,# 6,p. 457 - 461]
![[1,1'-Bicyclohexyl]-1-ol, 1'-(phenylseleno)-](/CAS/20210305/GIF/92514-27-7.gif)
92514-27-7
0 suppliers
inquiry
![Spiro[5.6]dodecan-7-one](/CAS/GIF/4728-90-9.gif)
4728-90-9
7 suppliers
inquiry
2888-11-1
7 suppliers
inquiry
![Spiro[5.6]dodecan-7-one](/CAS/GIF/4728-90-9.gif)
4728-90-9
7 suppliers
inquiry