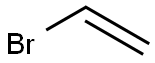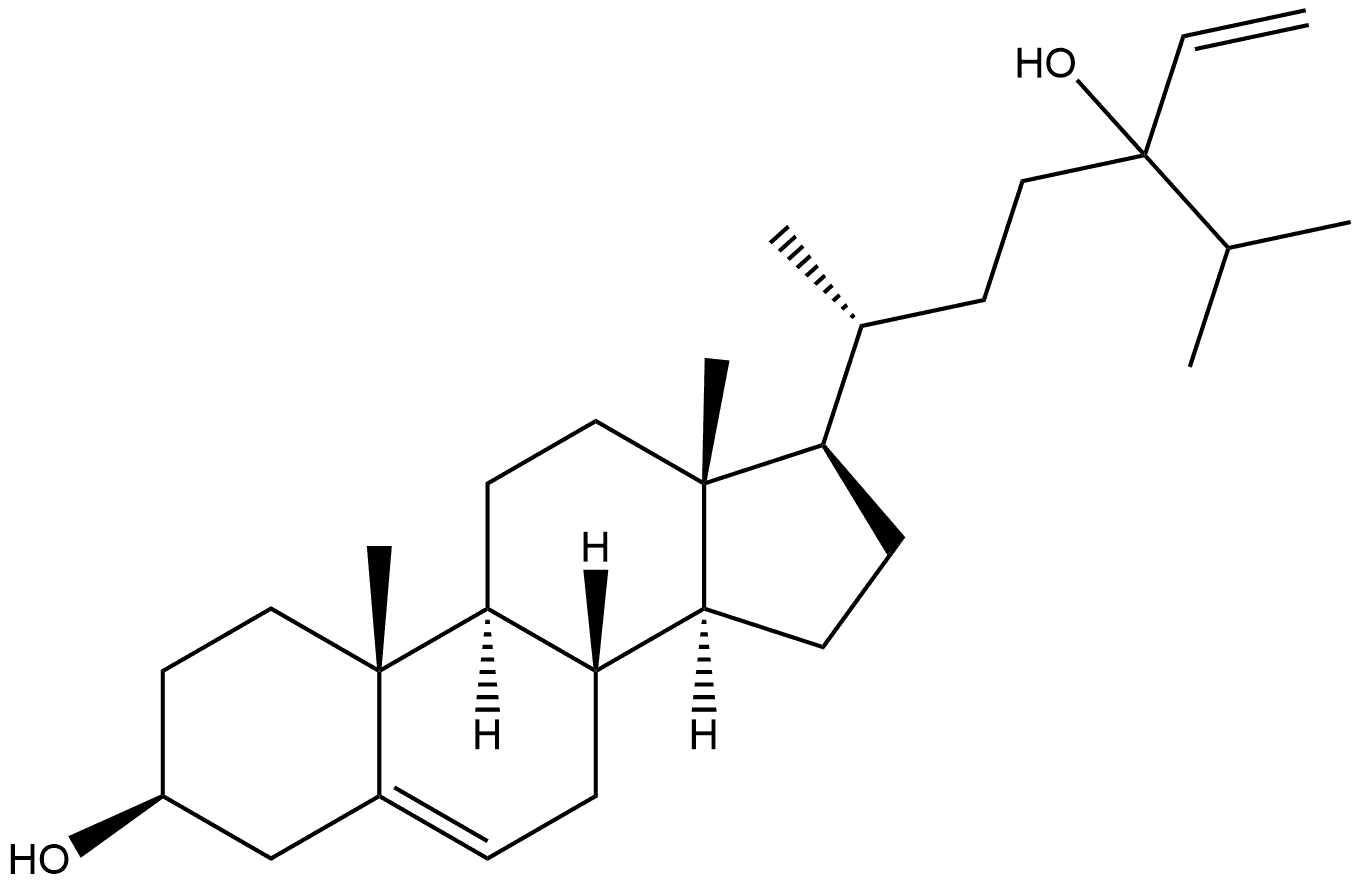
Stigmasta-5,28-diene-3,24-diol, (3β,24ξ)- synthesis
- Product Name:Stigmasta-5,28-diene-3,24-diol, (3β,24ξ)-
- CAS Number:934005-71-7
- Molecular formula:C29H48O2
- Molecular Weight:428.69

17752-16-8
10 suppliers
inquiry
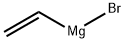
1826-67-1
211 suppliers
$60.89/100ml

934005-71-7
0 suppliers
inquiry
Yield:934005-71-7 90.8%
Reaction Conditions:
in tetrahydrofuran at 0 - 20;in;Sealed tube;Inert atmosphere;
Steps:
1.6 (6) Synthesis of sarigosterol (1)
Weigh compound 7 (3.2 g, 7.9 mmol) into a 250 mL dry three-neck flask, add a magnet, and seal with nitrogen. 0 °C ice bath, THF (50 mL) was added, after the solid was dissolved, vinylmagnesium bromide solution (30 mL, 30.0 mmol) was added dropwise with a dropping funnel, stirred at room temperature overnight, detected by TLC, and the raw material disappeared after 12 h. Drop to 0 °C, add saturated NH4Cl solution (50 mL) to the bottle and stir for 30 min to completely quench the Grignard reagent. After the reaction solution was concentrated by rotary evaporation, it was extracted with ethyl acetate (3×50 mL), then the ester layer was washed with saturated NaHCO3 solution (3×150 mL) and saturated NaCl solution (3×150 mL), dried over anhydrous NH/O4, and evaporated. Solvent, the crude product was purified by silica gel flash column chromatography (petroleum ether-ethyl acetate=90:10-50:50, v/v) to obtain compound 1 (white solid, 3.063 g) in a yield of 90.8%.
References:
CN113861258,2021,A Location in patent:Paragraph 0022; 0032-0033
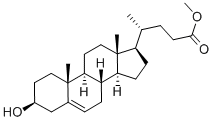
20231-57-6
26 suppliers
$225.00/100MG

934005-71-7
0 suppliers
inquiry

570-84-3
11 suppliers
$175.00/5mg

934005-71-7
0 suppliers
inquiry
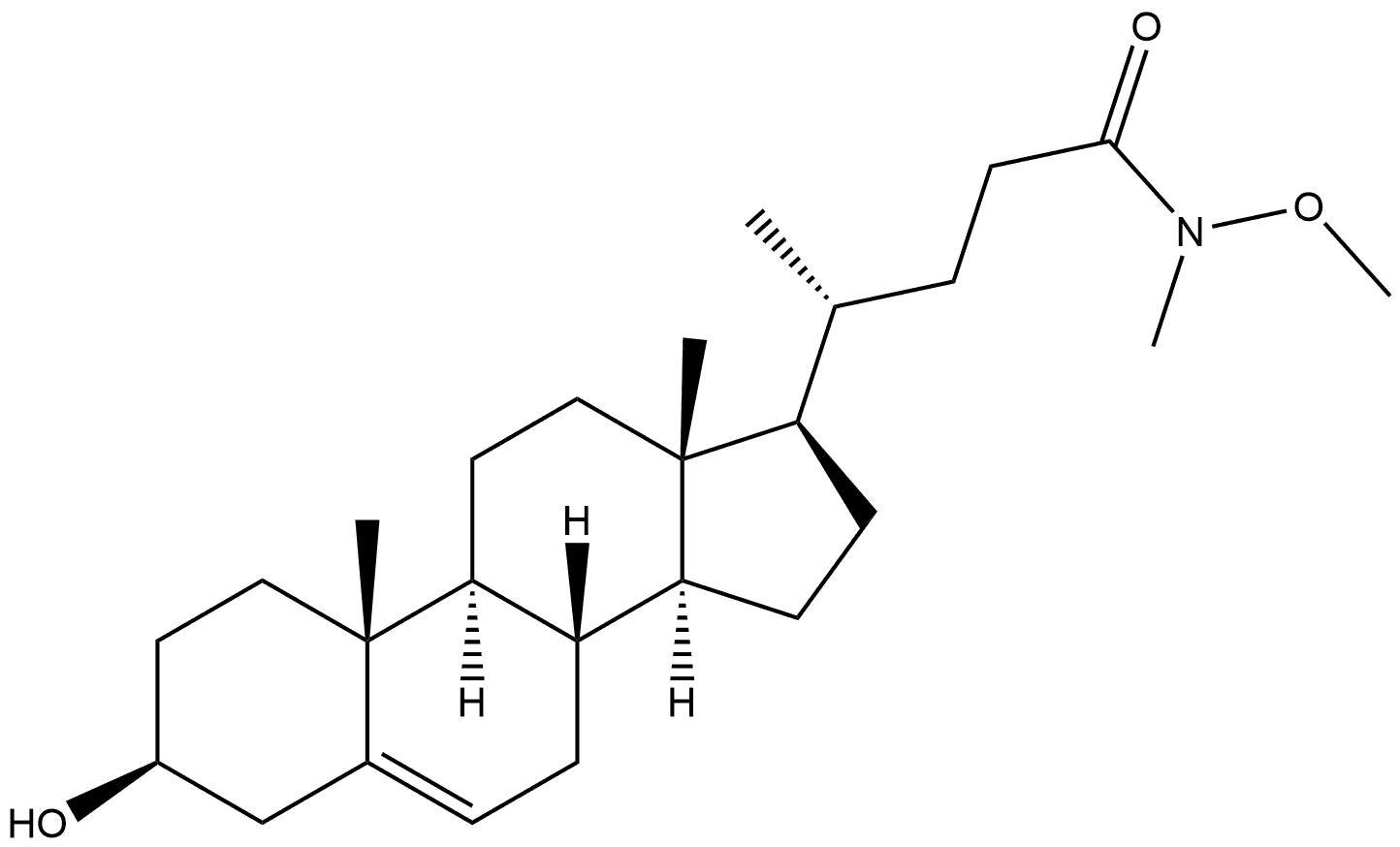
219903-24-9
0 suppliers
inquiry

934005-71-7
0 suppliers
inquiry