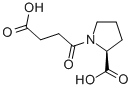
SUC-PRO-OH synthesis
- Product Name:SUC-PRO-OH
- CAS Number:63250-32-8
- Molecular formula:C9H13NO5
- Molecular Weight:215.2

108-30-5
614 suppliers
$5.00/25g
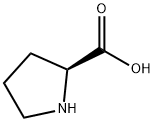
147-85-3
982 suppliers
$6.00/25g

63250-32-8
31 suppliers
$45.00/50mg
Yield:63250-32-8 46%
Reaction Conditions:
Stage #1: L-prolinewith triethylamine in acetonitrile at 0; for 0.25 h;
Stage #2: succinic acid anhydride in acetonitrile at 0; for 2 h;
Steps:
General procedure for the preparation of 1-(3-carboxypropanoyl)pyrrolidine-2-carboxylic acid(13)
General procedure: Triethylamine (1.2 mL, 8.7 mmol) was added to a supension of proline (3) (500 mg, 4.3 mmol) in dryacetonitrile (16 mL) at 0 °C and the mixture was stirred for 15 minutes. A solution of succinicanhydride (435 mg, 4.3 mmol) in dry acetonitrile was added dropwise at 0 °C. The mixture wasstirred for 2 hours at 0 °C then the solvent was removed under vacuum and ethyl acetate (10 mL) was added to the residue. The product was extracted with HCl solution (10 mL, 0.1 M), then the productwas re-extracted from the aqueous phase with ethyl acetate (6×40 mL). The combined extracts weredried (Na2SO4) and evaporated under reduced pressure to give a gum. This was dried by successivedissolution and evaporation from absolute ethanol (4×6 mL) and dichloromethane (5×6 mL) to givethe desired product as a white hygroscopic foam.
References:
Yusof, Yusralina;Tan, Daniel T.C.;Arjomandi, Omid Khalili;Schenk, Gerhard;McGeary, Ross P. [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 6,p. 1589 - 1593] Location in patent:supporting information