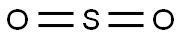
Sulfur dioxide synthesis
- Product Name:Sulfur dioxide
- CAS Number:7446-09-5
- Molecular formula:O2S
- Molecular Weight:64.06
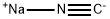
773837-37-9
0 suppliers
inquiry

67641-28-5
19 suppliers
$45.00/50mg

12769-73-2
0 suppliers
inquiry
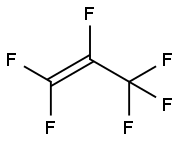
116-15-4
175 suppliers
$40.00/25g
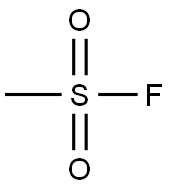
558-25-8
0 suppliers
$20.60/5g
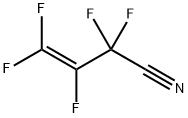
7792-66-7
5 suppliers
inquiry
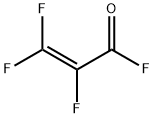
667-49-2
3 suppliers
inquiry
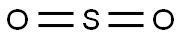
7446-09-5
137 suppliers
$195.00/25ML
Yield:7792-66-7 27%
Reaction Conditions:
in acetonitrile at -10 - 4.4; for 4.5 h;Inert atmosphere;Temperature;
Steps:
4.2.1. Method A
A 500-mL three-necked round-bottom flask, equipped with a magnetic stirrbar, reflux condenser, a dropping funnel, thermometer, and a bubble counter was charged with NaCN (42.5?g, 0.87?mol) suspended in MeCN (211?g, 245?mL) in the atmosphere of dry nitrogen. The suspension was cooled to -10?°C and PFAFS 1 (100.3?g, 0.44?mol) was slowly added within 2.5?h. At the same time, a strong exothermic was observed and the temperature raised up to 4.4?°C. In this case the addition of 1 immediately ceased and the temperature of the reaction mixture decreased again to -10?°C. After addition of 1, the reaction mixture was stirred for 2?h at -10?°C and afterwards it was distilled under reduced pressure. The following fractions were collected: fraction 1 (19.9?g,??50?°C/293?mmHg). Then, the fraction 1 was additionally distilled with a short Vigreux column. The fraction with b.p. 26-27?°C/760?mmHg was collected. 2 was obtained in 27% yield (18.6?g) as a colorless liquid.
References:
Tverdomed, Sergey N.;Hirschberg, Markus E.;Pajkert, Romana;Hintzer, Klaus;R?schenthaler, Gerd-Volker [Journal of Fluorine Chemistry,2018,vol. 210,p. 65 - 69]
7757-83-7
727 suppliers
$13.50/250G
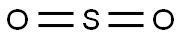
7446-09-5
137 suppliers
$195.00/25ML

75-15-0
261 suppliers
$40.00/100g
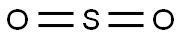
7446-09-5
137 suppliers
$195.00/25ML
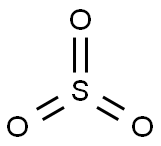
7446-11-9
69 suppliers
$662.15/1G
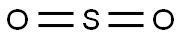
7446-09-5
137 suppliers
$195.00/25ML
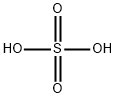
7664-93-9
0 suppliers
$23.30/1090731000
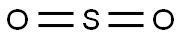
7446-09-5
137 suppliers
$195.00/25ML