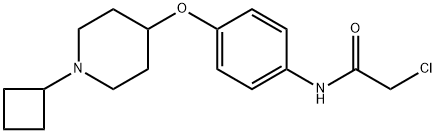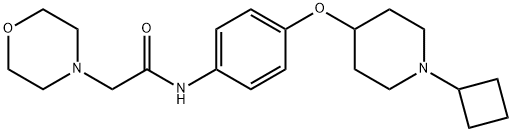
SUVN-G3031 synthesis
- Product Name:SUVN-G3031
- CAS Number:1394808-82-2
- Molecular formula:C21H31N3O3
- Molecular Weight:373.49
Yield:1394808-82-2 100%
Reaction Conditions:
Stage #1: 2-chloro-N-[4-(1-cyclobutyl piperidin-4-yloxy)phenyl]acetamidewith potassium carbonate in acetonitrile at 25 - 30; for 0.166667 h;Inert atmosphere;Large scale;
Stage #2: morpholine at 25 - 82; for 4 h;Reflux;Large scale;
Steps:
1.v Step (v): Preparation of N-[4-(1-cyclobutyl piperidin-4-yloxy) phenylj-2- (morpholin-4-yl) acetamide
Step (v): Preparation of N-[4-(1-cyclobutyl piperidin-4-yloxy) phenylj-2- (morpholin-4-yl) acetamideAcetonitrile (141 L) was charged into the GLR at 25-30 °C under nitrogen atmosphere, followed by addition of the above obtained material (9.4 Kg, 29.11M). Then, charged anhydrous K2C03 granules (6.0 Kg, 43.41 M) into the reactor at 25-30 °C. Stirred the reaction mass in the reactor for 10 minutes and charged morpholine (3.3 Kg, 37.88 M). The contents of the reactor were stirred for 15 minutes at 25-3 0 °C. The temperature of the reaction mass was raised slowly, to reflux (80-82 °C) and maintained at reflux for 4 hours while monitoring theprogress of the reaction every two hours by HPLC.Analysis of the sample by HPLC after 4 hours reflux: 89.61 % product and 8.83 % starting material (SM).Charged morpholine (253 grams) and K2C03 (400 grams) and further refluxed. Analysis by of the sample at 7.5 hours: 92.8 % product and 5.63 % SM.So charged morpholine (506 grams), K2C03 (810 grams) and acetonitrile (30 L) and heated the mass at reflux for another five hours. Analysis of the sample at 12.5 hours: 96.78 % product and 2.06 % SM. Again charged K2C03 (820 grams), morpholine (255 gm) and acetonitrile (40 L) and maintained the mass under reflux. Analysis of the sample at 19.5 hours: 97.52 % product and 0.9 % SM. Thereaction mass was cooled to 3 0-35 ?C.. and filtered solids through nutsche at 30-3 5°C. The cake on the nutsche was washed with 15 L acetonftrile Mother liquors (‘210 L filtrate) were taken back into the main reactor (GLR) and kept under stirring at 30 - 35 °C, while workup of the solid cake (22.4 Kg), containing the product along with salts, was going on in another reactor.Wet weight of cake: 22.4 Kg (contained 23 % product).Charged 30 L water into another reactor followed by the wet cake obtainedafter nutsche filtration (22.4 Kg). Stirred the mass for 30 minutes and charged EtOAc (47 L). The mass was stirred 15 minutes and settled for 15 minutes. The organic layer containing the product was collected in dedicated containers. pH ofthe aqueous mother liquors was found to be 10.05 on pH meter.2 extraction: Charged the above obtained aqueous layer into the reactor followed by EtOAc (47 L). The mass was stirred 15 minutes and settled for 15 minutes and layers separated. The organic layer containing the product was collected in dedicated containers.3nd extraction: Charged the above obtained aqueous layer into the reactorfollowed by EtOAc (40 L). The mass was stirred 15 minutes and settled for 15 minutes and layers separated. The organic layer containing the product was collected in dedicated containers.The combined organic layer was dried over sodium sulfate (9.4 Kg) andthe clean organic layer was taken for distillation under reduced pressure (> 500mm Hg) at 50-55 °C. The mass was cooled to 25-30 °C. Added 23.5 L of acetonitrile and stirred well.Part of the reaction mass (65 L of acetonitrile solution) from GLR was unloaded and charged into the above reaction mass at 25-30 °C and stirred 30minutes, whereby a clear solution was obtained. The mass was transferred to the main reactor. Washing was given to this reactor with 20 L fresh acetonitrile at 40- 45 °C and again transferred to the main reactor and stirred 15 minutes before sampling.The final, uniformly mixed reaction mass was sampled from the mainGLR and analyzed. HPLC: 99.09 % product and 0.31 % SM. So chargedmorpholine (510 grams) and K2C03 (825 grams) and the mass was heated to reflux and further maintained the mass at reflux temperature for 2 hours. A sample was analyzed after 2 hours reflux. Starting material was absent (product purity:99.24 %).The reflux was further continued for another 2 hours and then cooled the mass temperature to 30-3 5 °C. Solvent was distilled off under reduced pressure (> 500 mm Hg), maintaining mass temperature below 55 °C.1 Extraction: Charged DM water (23.5 L) to the residual mass at 25-30 °C.Stirred the mass for 15 minutes and charged ethyl acetate (80 L). A clear solutionwas obtained. Stirred the mass for 15 minutes and settled the mass for 15 minutes.Layers separated and the product organic layer collected in dedicated containers. 2nd Extraction: The aqueous layer obtained as above (pH was found to be 9.9 onmeter) was charged into the reactor followed by ethyl acetate (40 L). Stirred the mass for 15 minutes and settled the mass for 15 minutes. Layers separated and the product organic layer collected in dedicated containers.3nd Extraction: The aqueous layer obtained as above was once again charged intothe reactor followed by ethyl acetate (40 L). Stirred themass for 15 minutes and settled the mass for 15 minutes. Layers separated and the product organic layer collected in dedicated containers.Brine washing: The combined organic layer was taken in the reactor and charged 35 L brine solution (prepared by dissolving 9.4 Kg sodium chloride in 28.2 LDM water). The mass was stirred for 15 minutes and settled for 30 minutes. Layers separated and collected aqueous layer in dedicated containers.The organic product layer was dried over anhydrous sodium sulfate (18.8 Kg). Total volume of the organic layer was 185 L. The solvent was distilled off under reduced pressure (> 500 mm Hg) maintaining mass temperature below 55°C. Solid mass (Step-5 material) separated in reactor.Yield: Quantitative;Purity: 99.51 %;‘H-NMR (CDCI3, ? ppm): 1.65 - 2.04 (l2H, m), 2.61 - 2.63 (6H, m), 2.69 - 2.77 (1H, m), 3.12 (2H, s), 3.76-3.78 (4H, m), 4.26 -4.27 (1H, m), 6.87- 6.89 (2H, d,J = 8.82 Hz), 7.43 - 745 (2H, d, J = 8.80 Hz), 8.91 (1H, s); Mass (mi’z): 374.4 (M+H).
References:
WO2016/27275,2016,A1 Location in patent:Page/Page column 13; 14; 15
5382-16-1
13 suppliers
$9.00/5G

1394808-82-2
12 suppliers
inquiry
916905-62-9
1 suppliers
inquiry

1394808-82-2
12 suppliers
inquiry
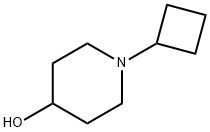
869224-62-4
31 suppliers
$130.00/250MG

1394808-82-2
12 suppliers
inquiry
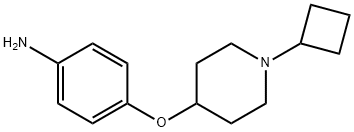
862310-33-6
8 suppliers
inquiry

1394808-82-2
12 suppliers
inquiry