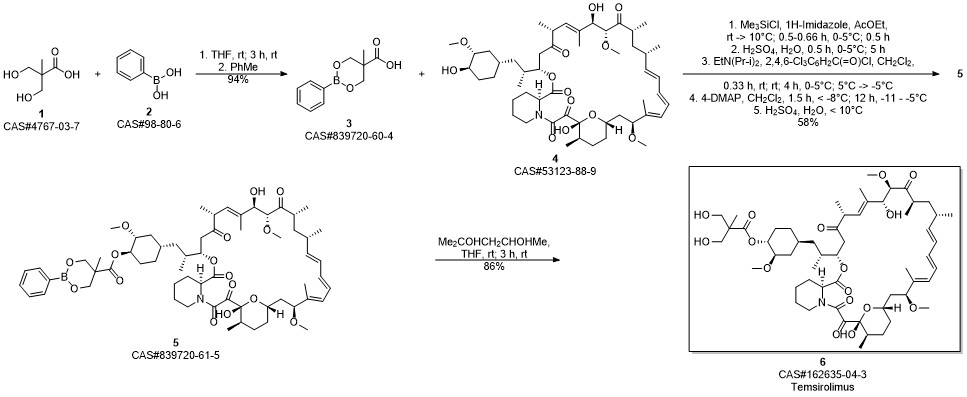
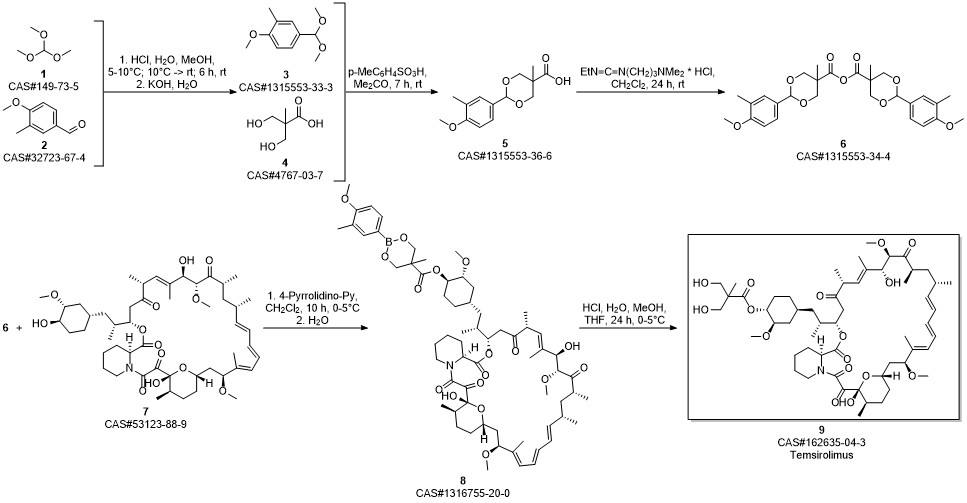
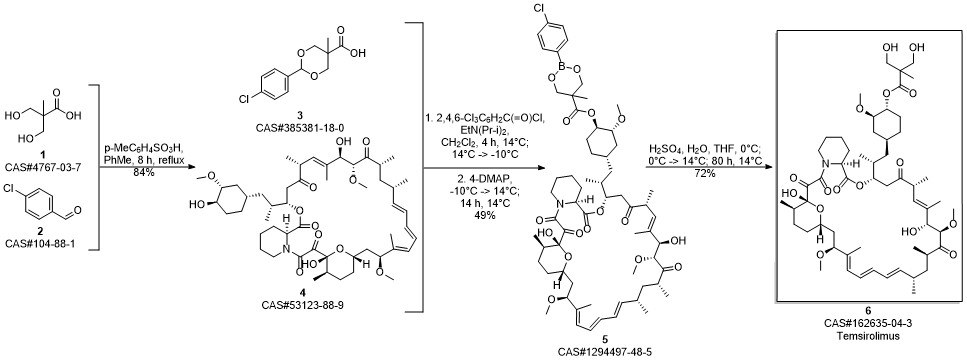
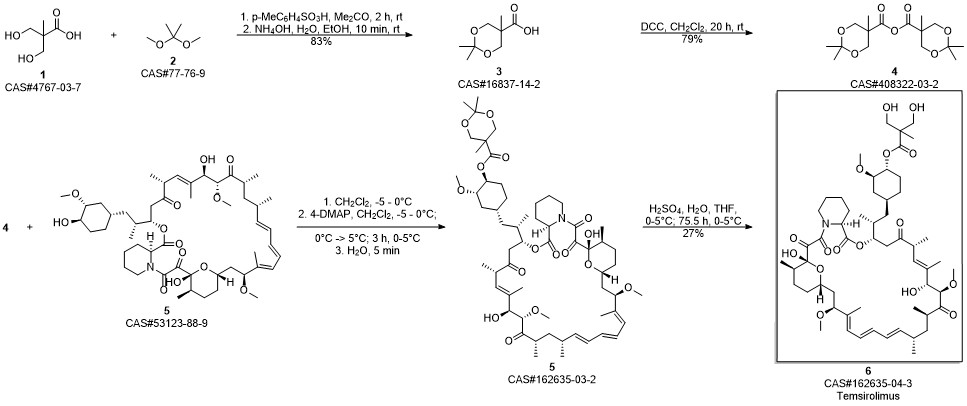
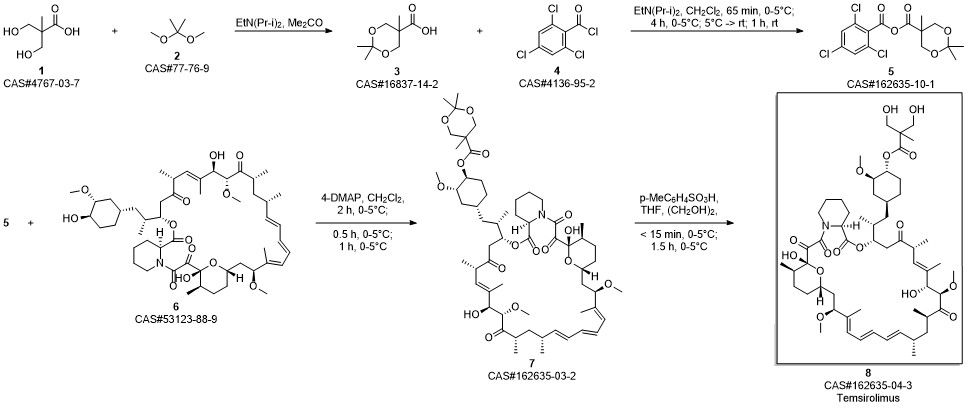
Temsirolimus synthesis
- Product Name:Temsirolimus
- CAS Number:162635-04-3
- Molecular formula:C56H87NO16
- Molecular Weight:1030.29
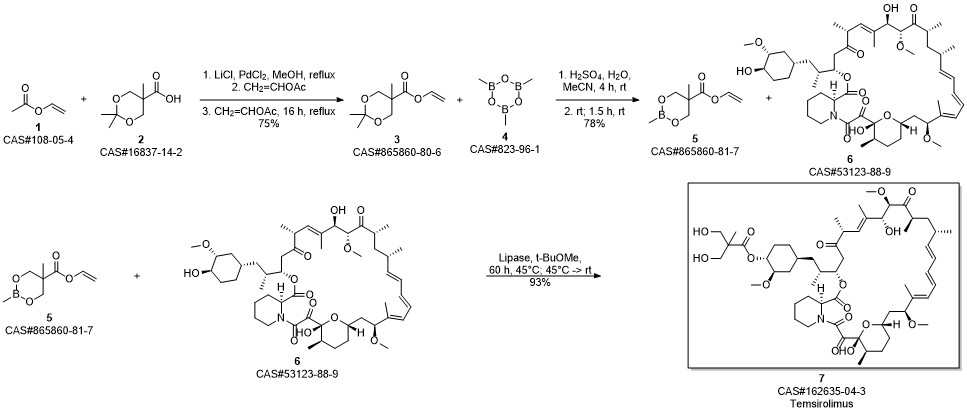
Gu, Jianxin; Ruppen, Mark E.; Raveendranath, Panolil; Chew, Warren; Shaw, Chia-Cheng. Production of rapamycin 42-ester with 2,2-bis(hydroxymethyl)propionic acid (CCI-779) and its proline analog. Assignee Wyeth, USA. US 20050234086. (2005).
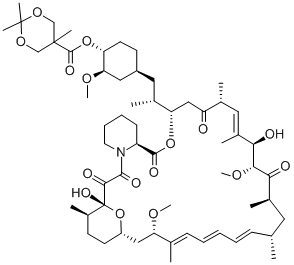
162635-03-2
14 suppliers
$250.00/25mg

162635-04-3
260 suppliers
$45.00/1mg
Yield:162635-04-3 83.2%
Reaction Conditions:
with sulfuric acid;water in tetrahydrofuran at 0 - 5;
Steps:
2
In a 5L glass reaction bottle,Add coupling intermediate 48g, 1000ml tetrahydrofuran,Stirring temperature control is 0~5 °C,Slowly add 126ml of 2mol/L sulfuric acid solution.After the drop, the temperature is stirred at 0 to 5 ° C,TLC detects the progress of the reaction and the reaction is completed.After that, 500 ml of drinking water was added to the reaction solution.1000ml of ethyl acetate, stirring, liquid separation,Ethyl acetate500ml/time extraction twice,Combine the organic layers with 1000 ml of saturated sodium bicarbonate solution,1000ml saturated saline solutionWashing, drying anhydrous sodium sulfate 300g for 3 to 4 hours; filtering, evaporating ethyl acetate to dryness under reduced pressure to give a pale yellow foamy solid;The yield was 83.2%.
References:
Lunan Pharmaceutical Group Co., Ltd.;Zhang Guimin;Bai Wenqin;Song Chuanling;Sun Xiuling CN108948046, 2018, A Location in patent:Paragraph 0062; 0063; 0068
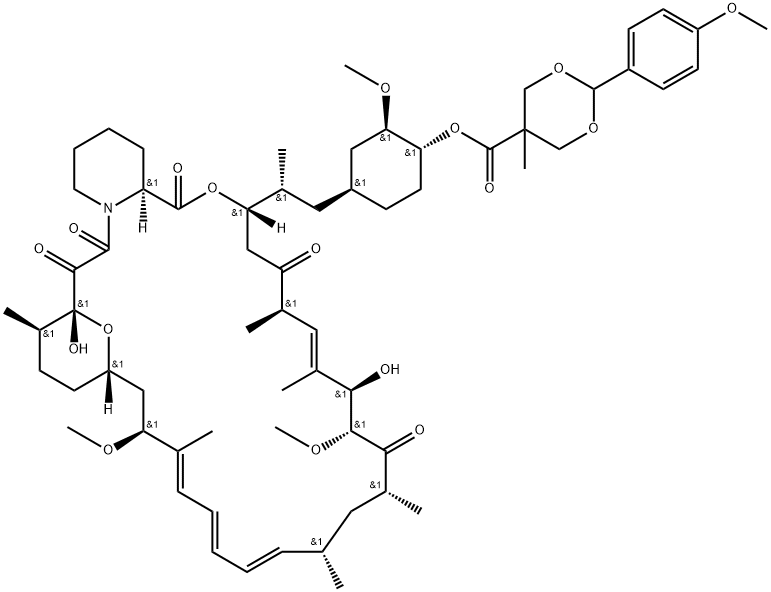
1316755-19-7
1 suppliers
inquiry

162635-04-3
260 suppliers
$45.00/1mg