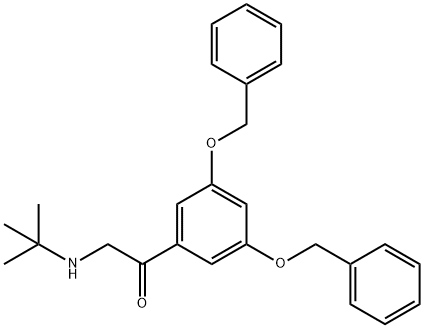
Terbutaline Impurity synthesis
- Product Name:Terbutaline Impurity
- CAS Number:52144-90-8
- Molecular formula:C26H29NO3
- Molecular Weight:403.51
![1-[3,5-bis(phenylmethoxy)phenyl]-2-bromoethan-1-one](/CAS/GIF/28924-18-7.gif)
28924-18-7
48 suppliers
$110.50/50mg
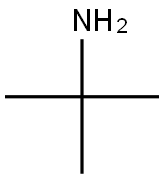
75-64-9
423 suppliers
$10.00/10g

52144-90-8
22 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen bromide in ethanol;benzene;
Steps:
1.f f.
f. 3,5-Dibenzyloxy-ω-(t-butylamino)-acetophenone 1.5 g of t-butylamine and 85 ml of absolute ethanol were placed in a 250 ml flask provided with a reflux condenser and the mixture was heated to reflux. The reaction was performed under nitrogen. At the reflux temperature, 4.1 g of 3,5-dibenzyloxy-ω-bromoacetophenone in 15 ml of benzene were added. The mixture was refluxed for twenty hours and after evaporation a yellow oil was recovered. This oil was shaken with absolute ether and white crystals were formed (t-butylamine hydrobromide). The crystals were filtered off and washed with ether. To the combined ether phases 10% hydrobromic acid was added and a white precipitate was formed. This precipitate was filtered off, thoroughly washed with water and ether, and recrystallized from ethanol: M.p. 196°-198°C.
References:
US3937838,1976,A