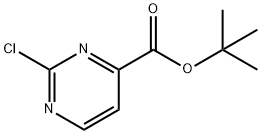
tert-Butyl 2-chloropyrimidine-4-carboxylate synthesis
- Product Name:tert-Butyl 2-chloropyrimidine-4-carboxylate
- CAS Number:220041-42-9
- Molecular formula:C9H11ClN2O2
- Molecular Weight:214.65
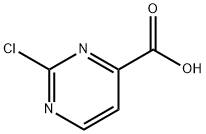
149849-92-3
192 suppliers
$6.00/250mg
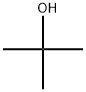
75-65-0
741 suppliers
$10.00/10ml

220041-42-9
30 suppliers
inquiry
Yield:220041-42-9 78%
Reaction Conditions:
with pyridine;p-toluenesulfonyl chloride at 20; for 16 h;Inert atmosphere;
Steps:
15A 15A. tert-Butyl 2-chloropyrimidine-4-carboxylate
In a 100 mE round-bottomed flask under nitrogen, pyridine (3 mE, 37.1 mmol) and p-toluenesulfonyl chloride (1.196 g, 6.27 mmol) were added to a suspension of 2-chlo- ropyrimidine-4-carboxylic acid (0.501 g, 3.16 mmol) in tert-butanol (20 mE, 209 mmol) and the mixture was stirred at room temperature for 16 h. The resulting solution was neutralized by slow addition of saturated aqueous sodium bicarbonate and the mixture was then concentrated in vacuo. The addition of water gave a precipitate which was filtered and the filter-cake was washed with watet The wet solid was dried by lyophilization to give tert-butyl 2-chloropyrimi- dine-4-carboxylate (0.526 g, 78%) as a light beige solid. EC (Analytical Method A): 1.735 mm. ‘H NMR (400 MHz, CDC13) 0 8.83 (d, J=5.1 Hz, 1H), 7.85 (d, J=4.7 Hz, 1H), 1.64 (s, 9H).
References:
US9598419,2017,B1 Location in patent:Page/Page column 56