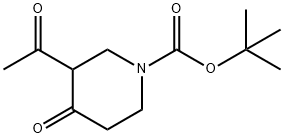
TERT-BUTYL 3-ACETYL-4-OXOPIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-ACETYL-4-OXOPIPERIDINE-1-CARBOXYLATE
- CAS Number:157327-43-0
- Molecular formula:C12H19NO4
- Molecular Weight:241.28

79099-07-3
543 suppliers
$5.00/5g

108-24-7
5 suppliers
$14.00/250ML

157327-43-0
21 suppliers
$300.00/100mg
Yield:157327-43-0 95%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-onewith pyrrolidine in 1,4-dioxane; for 3 h;Reflux;Inert atmosphere;
Stage #2: acetic anhydride in 1,4-dioxane;water;toluene at 20;Inert atmosphere;Reflux;
Steps:
60
Example 60:Tert-butyl 3-acetyl-4-oxopiperidine-1 -carboxylateThe compound of example 59 (50 g, 251 mmol) and pyrrolidine (41 .5 mL,502 mmol) were dissolved in dioxane (50 mL) and the resulting mixture wasref luxed for 3 hunder nitrogen using a Dean and Stark apparatus. The reaction mixture was then cooled to RT and concentrated. The residue was dissolved in toluene (250 mL), then acetic anhydride (52.1 mL, 552 mmol) was added and the resulting mixture was stirred under nitrogen. Water (60 mL) was added and theresulting mixture was refluxed for 1 h; then cooled to RT and concentrated. The residue was dissolved in water (100 mL) and the aqueous phase was extracted twice with ethyl acetate (100 mL).The combined organic extracts were washed with 5 % w/w HCI solution (50 mL) and dried over anhydrous sodium sulphate.The organic extract was concentrated to yield the title compound.Yield: 59.2 g (95 %); 1H NMR (DMSO-d6, 300 MHz): 64.19 (5, 2H), 3.73 (t, J= 6.0Hz, 1H), 3.59 (t, J= 6.0 Hz, 2H), 2.45 (t, J= 6.0 Hz, 2H), 2.14 (5, 3H), 1.49 (5, 9H);MS (ESl+): m/z 242.2 [M+H] HPLC Purity: 97.19%.
References:
WO2015/110999,2015,A1 Location in patent:Page/Page column 87; 88
2466-76-4
359 suppliers
$13.00/5g

79099-07-3
543 suppliers
$5.00/5g

157327-43-0
21 suppliers
$300.00/100mg

79099-07-3
543 suppliers
$5.00/5g

75-36-5
560 suppliers
$17.92/100G

157327-43-0
21 suppliers
$300.00/100mg

108-24-7
5 suppliers
$14.00/250ML
207691-65-4
35 suppliers
$140.68/250mgs:

157327-43-0
21 suppliers
$300.00/100mg

75-36-5
560 suppliers
$17.92/100G
217807-98-2
2 suppliers
inquiry

157327-43-0
21 suppliers
$300.00/100mg