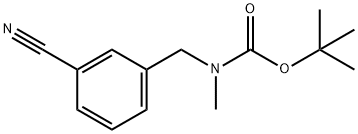
tert-Butyl 3-cyanobenzyl(methyl)carbamate synthesis
- Product Name:tert-Butyl 3-cyanobenzyl(methyl)carbamate
- CAS Number:1341536-23-9
- Molecular formula:C14H18N2O2
- Molecular Weight:246.3

24424-99-5
832 suppliers
$13.50/25G
90389-96-1
61 suppliers
$71.00/250mg

1341536-23-9
8 suppliers
inquiry
Yield:1341536-23-9 88%
Reaction Conditions:
in dichloromethane at 0 - 20; for 0.533333 h;Cooling with ice;
Steps:
42.2
Step 2: tert-butyl (3-cyanobenzyl)methylcarbamateIn a round bottom flask (100 ml), put under nitrogen atmosphere, a solution of crude 3-[(methylamino)methyl]benzonitrile (3.78 g; 23.79 mmol; 1 eq.) in DCM (37.80 ml) was prepared and cooled in an ice bath, then a solution of di-tert-butyl dicarbonate (5.19 g; 23.79 mmol; 1 eq.) in DCM (18.90 ml) was added over 2 minutes. The reaction mixture was stirred between 0°C and RT during 30 min. The reaction mixture was concentrated under reduced pressure to give a yellow oil (6.87 g). It was purified by flash chromatography (heptane / EtOAc gradient from 90:10 up to 70:30), affording the title compound as a colorless oil (5.13 g, 88%). 1H NMR (DMSO-de, 300 MHz) δ 7.76 (ddd, J = 6.9, 1 .9, 1 .8, 1 H), 7.65 (s, 1 H), 7.62 - 7.48 (m, 2H), 4.41 (s, 2H), 2.79 (s, 3H), 1 .62 - 1.16 (m, 9H). HPLC (Method A) Rt 4.28min (Purity: 100.0%).
References:
WO2012/4287,2012,A1 Location in patent:Page/Page column 94

28188-41-2
250 suppliers
$5.00/1g

1341536-23-9
8 suppliers
inquiry