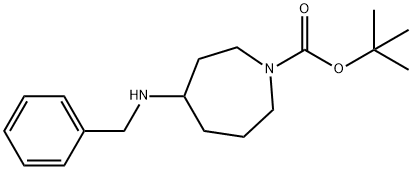
tert-butyl 4-(benzylaMino)azepane-1-carboxylate synthesis
- Product Name:tert-butyl 4-(benzylaMino)azepane-1-carboxylate
- CAS Number:878630-66-1
- Molecular formula:C18H28N2O2
- Molecular Weight:304.43
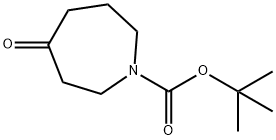
188975-88-4
262 suppliers
$6.00/250mg

100-46-9
463 suppliers
$5.00/5G

878630-66-1
9 suppliers
inquiry
Yield:878630-66-1 120 mg
Reaction Conditions:
Stage #1: tert-butyl 4-oxoazepane-1-carboxylate;benzylaminewith acetic acid in methanol at 25; for 0.5 h;
Stage #2: with sodium cyanoborohydride in methanol at 0 - 25; for 1.5 h;
Steps:
3.S-27 Step 1.
AcOH (53.6 μL, 0.94 mmol, 2.0 eq) was added to a solution of tert-butyl 4-oxoazepane-1-carboxylate (100.0 mg, 0.47 mmol, 1.0 eq) and BnNH2 (61.5 μL, 0.56 mmol, 1.2 eq) in MeOH (5 mL) at 25° C. The reaction was stirred for 30 min, after which NaBH3CN (44.2 mg, 0.70 mmol, 1.5 eq) was added at 0° C. and the mixture was stirred at 25° C. for additional 1.5 h. Upon completion, the reaction was quenched by the addition of water (10 mL) and extracted with DCM (3×5 mL). The combined organic layers were dried over Na2SO4 and concentrated to give crude compound SI-48 (120.0 mg) as yellow oil, which was used in step 2 without further purification.
References:
US2020/278355,2020,A1 Location in patent:Paragraph 0387; 0494-0495

188975-88-4
262 suppliers
$6.00/250mg
39110-74-2
31 suppliers
inquiry

878630-66-1
9 suppliers
inquiry