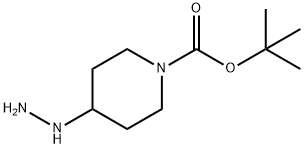
tert-Butyl 4-hydrazinopiperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-hydrazinopiperidine-1-carboxylate
- CAS Number:1196486-69-7
- Molecular formula:C10H21N3O2
- Molecular Weight:215.29

79099-07-3
543 suppliers
$5.00/5g

7803-57-8
4 suppliers
$20.10/50g

1196486-69-7
19 suppliers
inquiry
Yield: 88%
Reaction Conditions:
Stage #1:N-tert-butyloxycarbonylpiperidin-4-one;hydrazine hydrate in ethanol at 20;Inert atmosphere;
Stage #2: with sodium tetrahydroborate in methanol;ethanol at 0 - 20;
Steps:
9
Intermediate 9: 4-Hydrazino-piperidine-1-carboxylic acid tert-butyl ester; Hydrazine hydrate (100 mL, 2 mol) was added to a solution of 1-Boc-4-piperidone (available from Aldrich Chemical Company, Inc., Milwaukee, Wis., USA 20.0 g, 100 mmol) in ethanol (180 mL) under nitrogen at room temperature and the reaction mixture was stirred at room temperature overnight. Methanol (180 mL) was added and the reaction mixture was cooled to 0-5° C. Sodium borohydride (14.2 g, 375 mmol) was added in portions and the reaction mixture was allowed to warm to room temperature and stir for 2 h. The solvents were evaporated under reduced pressure, dichloromethane was added and the mixture was washed with water, dried (sodium sulfate), filtered, and evaporated to give 4-hydrazino-piperidine-1-carboxylic acid tert-butyl ester (18.9 g, 88%) as a viscous oil, which was used for the next step without further purification.
References:
Location in patent:Page/Page column 21

79099-07-3
543 suppliers
$5.00/5g

1196486-69-7
19 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1196486-69-7
19 suppliers
inquiry