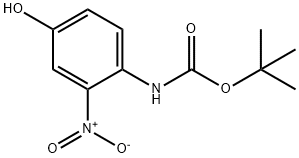
tert-butyl 4-hydroxy-2-nitrophenylcarbaMate synthesis
- Product Name:tert-butyl 4-hydroxy-2-nitrophenylcarbaMate
- CAS Number:201811-20-3
- Molecular formula:C11H14N2O5
- Molecular Weight:254.24

24424-99-5
826 suppliers
$13.50/25G
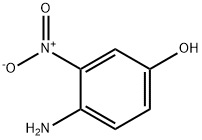
610-81-1
334 suppliers
$5.00/5g

201811-20-3
26 suppliers
inquiry
Yield: 95%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 25; for 2 h;
Steps:
8.1 Step 1 to synthesize compound 12
4-amino-3-nitro-phenol (1.5 g, 9.73 mmol), B0C2O (3.19 g, 14.60 mmol) and Et3N (984 mg, 9.73 mmol) dissolved in DCM (10 mL). Then a solution of DMAP (118 mg, 0.973 mmol) in DCM (10 mL) was slowly added dropwise to the above solution. Then the mixture was stirred at 25°C for 2 hours. The mixture was concentrated under reduced pressure to remove DCM. The residue was purified by silica gel chromatography. (ISCO; 20 g SepaFlash Silica Flash Column, Eluent of 0-30% Ethyl acetate/Petr oleum ether gradient 40 mL/min) to afford tert-butyl A-(4-hydroxy-2-nitro-phenyl (carbamate (2.35 g, 95% yield) as a yellow solid. LCMS: /R = 1.169 min in 10- 80AB_2min_220&254_Shimadzu.lcm, MS (ESI) m/z = 255.1 [M+H]+.
References:
BRIDGENE BIOSCIENCES, INC.;CAO, Ping;ZHANG, Chao;BISHOP, Michael, J. WO2020/168237, 2020, A1 Location in patent:Paragraph 00173; 00174
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

201811-20-3
26 suppliers
inquiry

610-81-1
334 suppliers
$5.00/5g

201811-20-3
26 suppliers
inquiry
215656-99-8
10 suppliers
inquiry

201811-20-3
26 suppliers
inquiry