
tert-Butyl 4-vinylcyclohexylcarbaMate synthesis
- Product Name:tert-Butyl 4-vinylcyclohexylcarbaMate
- CAS Number:1198355-16-6
- Molecular formula:C13H23NO2
- Molecular Weight:225.33
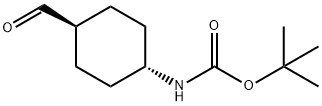
181308-57-6
101 suppliers
$32.00/100mg
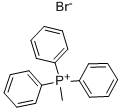
1779-49-3
598 suppliers
$5.00/10g

1198355-16-6
30 suppliers
$60.00/100mg
Yield:1198355-16-6 80%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran at 20; for 1 h;Inert atmosphere;
Steps:
B B. tert-Butyl ((trans)-4-vinylcyclohexyl)carbamate
To an ice-cold suspension of methyltriphenylphosphonium bromide (5.03 g, 14.08 mmol) in THF (25 mL), under N2, was added potassium tert-butoxide (1.580 g, 14.08 mmol) in portions. After 5 min, the ice bath was removed and the mixture was stirred at rt. After ~1 h, tert-butyl ((trans)-4-formylcyclohexyl)carbamate (1.6 g, 7.04 mmol) was added. The mixture was stirred at rt for ~1 h, and then, aq. satd. NH4CI solution (~10 mL) was slowly added. The mixture was partitioned between EtOAc and water. The aqueous phase was washed 1X with EtOAc. The combined organic phases were washed 1X with brine, dried over Na2S04, filtered, and concentrated. The residue was purified by silica gel chromatography, eluting with 0-50% EtOAc:hexanes gradient (fractions were checked with PMA stain) to give tert-butyl ((trans)-4-vinylcyclohexyl)carbamate (1.329 g, 5.60 mmol, 80% yield) as a white solid. 1H NMR (400 MHz, CD3SOCD3) d ppm 6.72 (d, J = 8 Hz, 1 H), 5.66- 5.86 (m, 1 H), 4.97 (dd, J = 17, 2 Hz, 1 H), 4.89 (dd, J = 10, 2 Hz, 1 H), 3.05-3.22 (m, 1 H), 1.74-1.94 (m, 3 H), 1 .65-1.72 (m, 2 H), 1.38 (s, 9 H), 1.00-1.27 (m, 4 H).
References:
WO2019/116256,2019,A1 Location in patent:Page/Page column 66; 67