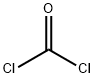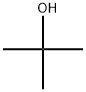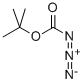
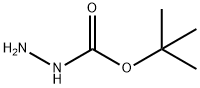
tert-butyl azidoformate synthesis
- Product Name:tert-butyl azidoformate
- CAS Number:1070-19-5
- Molecular formula:C5H9N3O2
- Molecular Weight:143.1439
The Direct Preparation of tert-Butyl Azidoformate
870-46-2
537 suppliers
$5.00/10g

1070-19-5
11 suppliers
inquiry
Yield:1070-19-5 97%
Reaction Conditions:
with potassium hydrogencarbonate;sodium nitrite in water;acetic acid
Steps:
4.a (a)
(a) t-Butyl azidoformate 60 g of t-butyloxycarbonylhydrazine are dissolved at about 30° in 48 ml of acetic acid; the solution is diluted with 72 ml of water and cooled to -2° with an ice-salt bath. 34.6 g of sodium nitrite, dissolved in 48 ml of water, are added dropwise to the mixture, whilst stirring magnetically and keeping the temperature in the region of 0°. The stirring is continued for 1 hour 30 minutes after the end of the addition, the mixture is diluted with 60 ml of water and the supernatant organic phase is decanted. The aqueous phase is extracted 3 or 4 times with pentane and these extracts are mixed with the 1st organic phase. The solution obtained is washed 5 times with 30 ml of water, 3 times with 60 ml of a N solution of potassium bicarbonate and finally 3 times with 60 ml of water. After drying over magnesium sulphate, the solvent is evaporated off at ordinary temperature. 59 g (yield 97%) of crude t-butyl azidoformate, which is used without purification, are obtained. This compound boils at 34°/12 mm Hg.
References:
Synthelabo US4134991, 1979, A
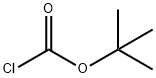
24608-52-4
17 suppliers
inquiry

1070-19-5
11 suppliers
inquiry

75-09-2
1196 suppliers
$10.00/25 mL

107-11-9
1 suppliers
$26.00/25ml

1070-19-5
11 suppliers
inquiry

78888-18-3
106 suppliers
$32.00/5g

24424-99-5
823 suppliers
$13.50/25G

1070-19-5
11 suppliers
inquiry