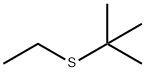
TERT-BUTYL ETHYL SULFIDE synthesis
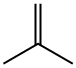
- Product Name:TERT-BUTYL ETHYL SULFIDE
- CAS Number:14290-92-7
- Molecular formula:C6H14S
- Molecular Weight:118.24
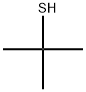
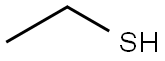
75-66-1
131 suppliers
$18.00/25mL
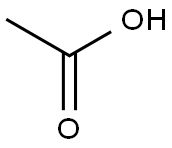
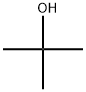
64-19-7
1535 suppliers
$10.00/25ML

14290-92-7
15 suppliers
inquiry
Yield:-
Reaction Conditions:
with indium (III) iodide;Hexamethyldisiloxane in 1,2-dichloro-ethane at 80; for 4 h;Inert atmosphere;
Steps:
General procedure for the synthesis of unsymmetrical sulfides
General procedure: To a freshly distilled 1,2-dichloroethane solution (0.6 mL) in a screw-capped tube under a N2 atmosphere were successively added a magnetic stirrer bar, an aliphatic carboxylic acid (0.75mmol), tBuSH (0.60 mmol, 54 mg), InI3 (0.060 mmol, 30 mg), and TMDS (1.8 mmol, 3.2×102μL). The reaction tube was heated at 80°C for 4 h. After cooling to room temperature, an aromatic carboxylic acid (0.75 mmol) and TMDS (1.8 mmol, 3.2×102μL) were then added to the reaction vial. The mixture was heated at 80°C for 20 h again. After the reaction, the reaction resultant mixture was quenched by a saturated NaHCO3 aqueous solution (3 mL). The aqueous layer was extracted with chloroform (3 mL×3). The combined organic phase was dried over anhydrous Na2SO4, filtered, and then evaporated under reduced pressure. The crude material was purified by column chromatography (silica gel, 99/1=hexane/EtOAc) to give the corresponding dialkyl sulfide. The ratio of the remaining intermediate was determined by NMR using 1,1,2,2-tetrachloroethane as an internal standard.
References:
Sakai, Norio;Yoshimoto, Shunsuke;Miyazaki, Takahiro;Ogiwara, Yohei [Tetrahedron Letters,2016,vol. 57,# 29,p. 3117 - 3120] Location in patent:supporting information
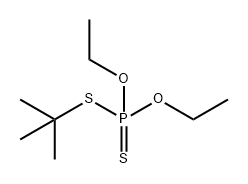
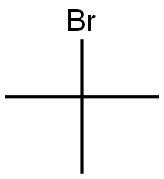
66427-05-2
0 suppliers
inquiry

14290-92-7
15 suppliers
inquiry