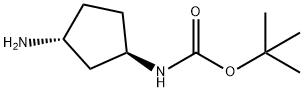
tert-butyl n-[(1r,3r)-3-aminocyclopentyl]carbamate synthesis
- Product Name:tert-butyl n-[(1r,3r)-3-aminocyclopentyl]carbamate
- CAS Number:1009075-44-8
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28
![Carbamic acid, N-[(1R,3R)-3-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopentyl]-, 2-propen-1-yl ester](/CAS/20210305/GIF/1315495-80-7.gif)
1315495-80-7
0 suppliers
inquiry
![tert-butyl n-[(1r,3r)-3-aminocyclopentyl]carbamate](/CAS/20180703/GIF/1009075-44-8.gif)
1009075-44-8
63 suppliers
inquiry
Yield:1009075-44-8 56%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);1,3-dimethylbarbituric acid in dichloromethane at 20; for 1.5 h;
Steps:
8 ((lR,3R)-3-Amino-cyclopentyl)-carbamic acid tert-butyl ester
A mixture of ((lR,3R)-3-allyloxycarbonylamino-cyclopentyl)-carbamic acid tert-butyl ester (890 mg, 3.13 mmol), 1,3-dimethylbarbituric acid (1.47 g, 9.39 mmol) and Pd(PPh3)4 (181 mg, 0.15 mmol) in DCM (30 mL) was stirred at ambient temperature for 90 minutes. The reaction mixture was concentrated under vacuum and purified by column chromatography on silica gel (gradient: 2M NH3 in methanol solution in DCM) to afford 350 mg (56%) ((lR,3R)-3-amino-cyclopentyl)- carbamic acid tert-butyl ester. LCMS (Method F, ESI): RT = 0.36 min, m+H = 200.9; 1H NMR (400 MHz, DMSO-d6) δ: 6.77 (s, 1 H), 3.90 (m, 1 H), 3.31 (m, 1 H), 1.87 (m, 2 H), 1.54 (m, 2 H), 1.37 (s, 10 H), 1.18 (m, 1 H).
References:
WO2013/7768,2013,A1 Location in patent:Page/Page column 118
489446-85-7
79 suppliers
$68.00/50mg
![tert-butyl n-[(1r,3r)-3-aminocyclopentyl]carbamate](/CAS/20180703/GIF/1009075-44-8.gif)
1009075-44-8
63 suppliers
inquiry