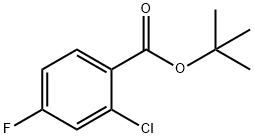
tert-Butyl2-chloro-4-fluoroBenzoate synthesis
- Product Name:tert-Butyl2-chloro-4-fluoroBenzoate
- CAS Number:911314-43-7
- Molecular formula:C11H12ClFO2
- Molecular Weight:230.66

21900-54-9
163 suppliers
$13.00/1g
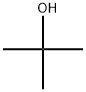
75-65-0
739 suppliers
$10.00/10ml

911314-43-7
36 suppliers
$25.00/250mg
Yield:911314-43-7 71 %
Reaction Conditions:
Stage #1: tert-butyl alcoholwith n-butyllithium in tetrahydrofuran at 0;Inert atmosphere;
Stage #2: 2-chloro-4-fluorobenzoyl chloride in tetrahydrofuran at 0;Inert atmosphere;
Steps:
19.19b
Step 19b: Preparation of tert-butyl 2-chloro-4-fluorobenzoate (Compound 0102-28): To 2-chloro-4-fluorobenzoic acid (2.0 g, 11.46 mmol, 1.0 equiv)To a mixture of N,N-dimethylformamide (2 drops) in dichloromethane (20 mL) was added oxalyl chloride (1.94 mL, 22.91 mmol, 2.0 equiv).The mixture was stirred at room temperature for 4 hours. The solvent was removed under reduced pressure,and the residue was dissolved in tetrahydrofuran (5 mL) to obtain2-Chloro-4-fluorobenzoyl chloride solution. At 0°C under a nitrogen atmosphere,To a mixture of tert-butanol (1.32 mL, 13.75 mmol, 1.2 eq) in THF (15 mL) was added n-butyllithium (2.5M in hexane, 5.04 mL, 12.61 mmol, 1.1 eq) dropwise. The mixture was stirred for 10 minutes.A tetrahydrofuran solution of 2-chloro-4-fluorobenzoyl chloride was added dropwise,The mixture was then stirred at 0 °C for 1 hour. The reaction was quenched by adding saturated ammonium chloride solution (30 mL). The aqueous layer was extracted with ethyl acetate (20 mL×3). The combined organic layers were washed with saturated brine (20 mL×1),Dried over anhydrous sodium sulfate and concentrated. The residue was subjected to column chromatography on silica gel (petroleum ether: ethyl acetate 50:1) to obtain pale yellow oiltert-butyl 2-chloro-4-fluorobenzoate (1.88 g, yield: 71%).
References:
WO2022/213932,2022,A1 Location in patent:Page/Page column 62-63
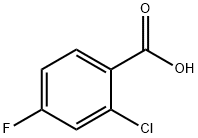
2252-51-9
368 suppliers
$7.00/5g

75-65-0
739 suppliers
$10.00/10ml

911314-43-7
36 suppliers
$25.00/250mg

2252-51-9
368 suppliers
$7.00/5g

911314-43-7
36 suppliers
$25.00/250mg