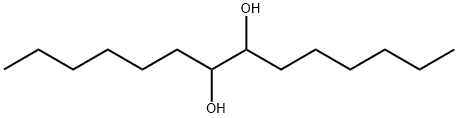
tetradecane-7,8-diol synthesis
- Product Name:tetradecane-7,8-diol
- CAS Number:16000-65-0
- Molecular formula:C14H30O2
- Molecular Weight:230.39
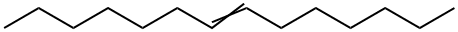
10374-74-0
44 suppliers
$95.00/5mL

16000-65-0
3 suppliers
inquiry
Yield:16000-65-0 88%
Reaction Conditions:
with formic acid;dihydrogen peroxide at 20 - 50;Cooling;
Steps:
2
EXAMPLE 2[0061 ] This Example serves to illustrate synthesis and isolation of a diol, and subsequent esterification of said diol, en route to synthesis of a diester species, in accordance with some embodiments of the present invention.Diol Preparation and Isolation[0062] In a 3-neck 1 mL reaction flask equipped with an overhead stirrer and an ice bath, 75 mL of 30% hydrogen peroxide were added to 300 mL of 96% formic acid. To this mixture, 100 grams (0.51 mole) of 7-tetradecene (purchased from Aldrich
References:
WO2009/142922,2009,A1 Location in patent:Page/Page column 14-15
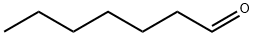
111-71-7
214 suppliers
$13.50/100ML

16000-65-0
3 suppliers
inquiry
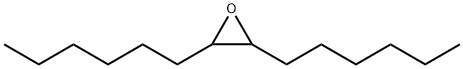
85721-27-3
1 suppliers
inquiry

16000-65-0
3 suppliers
inquiry

41446-63-3
12 suppliers
$361.00/5g

16000-65-0
3 suppliers
inquiry

41446-63-3
12 suppliers
$361.00/5g
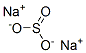
7757-83-7
695 suppliers
$13.50/250G

16000-65-0
3 suppliers
inquiry