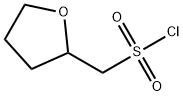
tetrahydro-2-Furanmethanesulfonyl chloride synthesis
- Product Name:tetrahydro-2-Furanmethanesulfonyl chloride
- CAS Number:338453-29-5
- Molecular formula:C5H9ClO3S
- Molecular Weight:184.64
![Furan, 2,2'-[dithiobis(methylene)]bis[tetrahydro- (9CI)](/CAS/20211123/GIF/14605-21-1.gif)
14605-21-1
0 suppliers
inquiry

338453-29-5
18 suppliers
inquiry
Yield:338453-29-5 56%
Reaction Conditions:
with hydrogenchloride;iodosylbenzene in diethyl ether;water at 20; for 1 h;
Steps:
42c.b Stage b) Tetrahydrofuran-2-yl-methanesulphonyl chloride
Dithiobis-(tetrahydrofuran-2-yl)-methan-yl (4.07 g; 32.6 mmol) is dissolved in ether (300 ml). iodosobenzene (42 g; 130 mmol) and hydrochloric acid (55 ml; 37%) are added at room temperature and the entire mixture is stirred for one hour. The reaction mixture is poured on to a saturated sodium carbonate solution and extracted twice with ether. The organic phases are dried over sodium sulphate, the salts are filtered off and the solvent is evaporated off. The residue is chromatographed over silica gel with n-hexanelethyl acetate (7/3). The pure fractions are combined and the solvent is evaporated off. [01226] Yield: 6.76 g (56%) tetrahydrofuran-2-yl-methanesulphonyl chloride as a slightly pale brown liquid. NMR (250 MHz; J in Hz; CDCl3)in ppm: 4.58 (m; 1H); 4.02-3.75 (m; 4H); 2.25 (m; 1H); 1.99 (m; 2H); 1.76 (m; 1H).
References:
US6821980,2004,B1 Location in patent:Page column 116-117
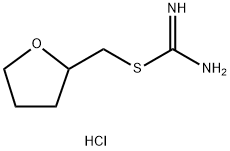
1062292-82-3
0 suppliers
inquiry

338453-29-5
18 suppliers
inquiry
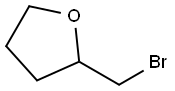
1192-30-9
168 suppliers
$10.00/1g

338453-29-5
18 suppliers
inquiry
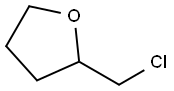
3003-84-7
170 suppliers
$53.00/100mg

338453-29-5
18 suppliers
inquiry