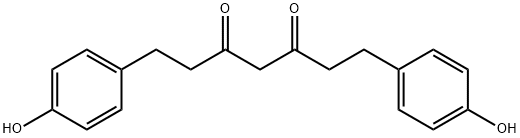
TETRAHYDROBISDEMETHOXYDIFERULOYLMETHANE synthesis
- Product Name:TETRAHYDROBISDEMETHOXYDIFERULOYLMETHANE
- CAS Number:113482-94-3
- Molecular formula:C19H20O4
- Molecular Weight:312.36
Yield:113482-94-3 45%
Reaction Conditions:
Stage #1: 4-(4-trimethylsilyloxyphenyl)butan-2-one;C12H17ClO2Siwith lithium diisopropyl amide in tetrahydrofuran at -78; for 0.583333 h;
Stage #2: with potassium carbonate in methanol; for 0.5 h;
Steps:
3
Compound (22) was treated with lithium diisopropylamide (LDA, 1.5 M in THF, 1. equiv) in tetrahydrofuran (THF) at -78° C. under N2 for 20 min and 1.1 equivalents of the TMS protected acyl chloride, compound (20), dissolved in THF was added. The reaction mixture was stirred at -78° C. for 15 minutes and slowly warmed to room temperature. The reaction mixture was quenched with water and poured into ethyl acetate. The organic layer was washed three times with water and the water layer was back washed (2×) with ethyl acetate. The organic layers were combined, dried (MgSO4), filtered, and the solvent was removed under vacuum. The residue was stirred in methanol in the presence of K3CO3 for 30 min to remove TMS protection. The solution was acidified with 2N HCl and poured into ethyl acetate. The aqueous layer was partitioned three times with ethyl acetate and the organic layers were combined, dried (MgSO4), filtered, and the solvent was removed under vacuum. The residue was column chromatographed over silica gel using a gradient elution of ethyl acetate/petroleum ether to afford compound (9). Compound (9) was further purified using semi-preparative HPLC using an acetonitrile/water (90/10) solvent system to give pure synthesized curcuminoid compound (9) in 45% overall yield. Similarly, the unsymmetric synthesized curcuminoid compound (4) was prepared in 40% overall yield.
References:
US6887898,2005,B1 Location in patent:Page/Page column 19; sheet 2
52328-96-8
22 suppliers
inquiry

113482-94-3
4 suppliers
inquiry
123-54-6
567 suppliers
$10.00/25ml

113482-94-3
4 suppliers
inquiry

123-08-0
892 suppliers
$5.00/10g

113482-94-3
4 suppliers
inquiry
![2-Butanone, 4-[4-[(trimethylsilyl)oxy]phenyl]-](/CAS/20210305/GIF/522617-84-1.gif)
![Benzenepropanoyl chloride, 4-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/896460-66-5.gif)